Abstract
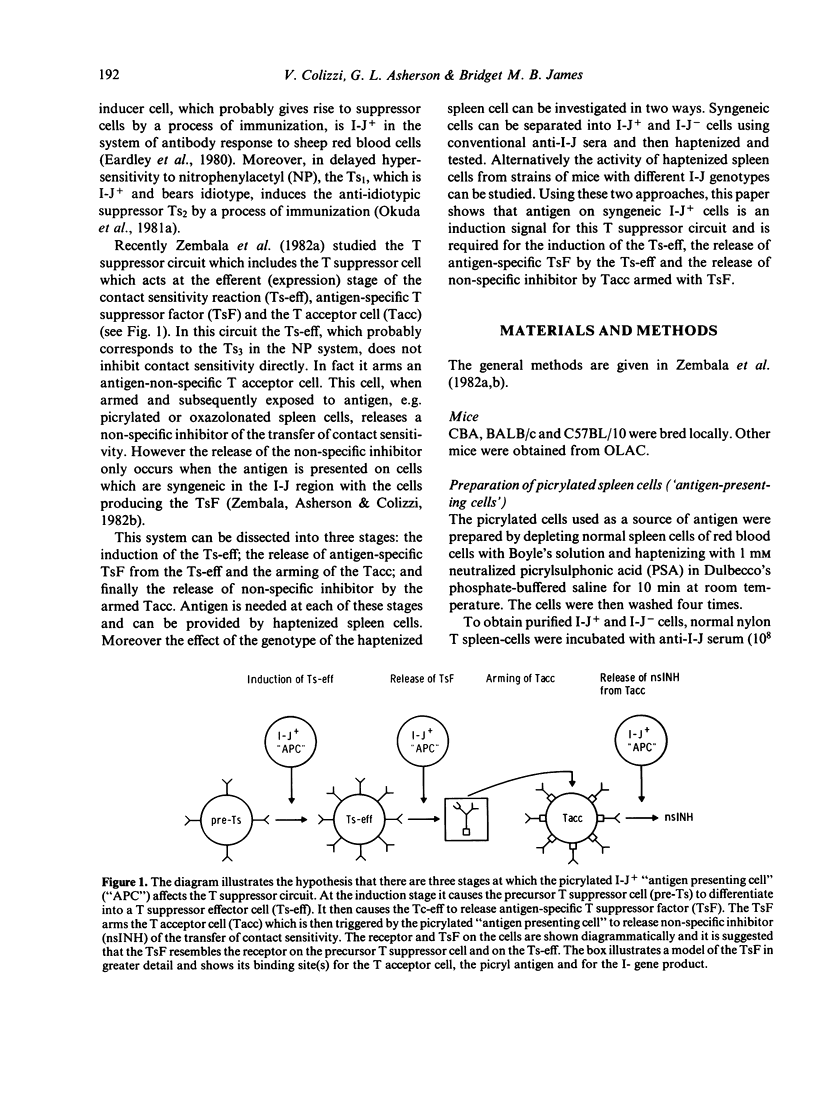
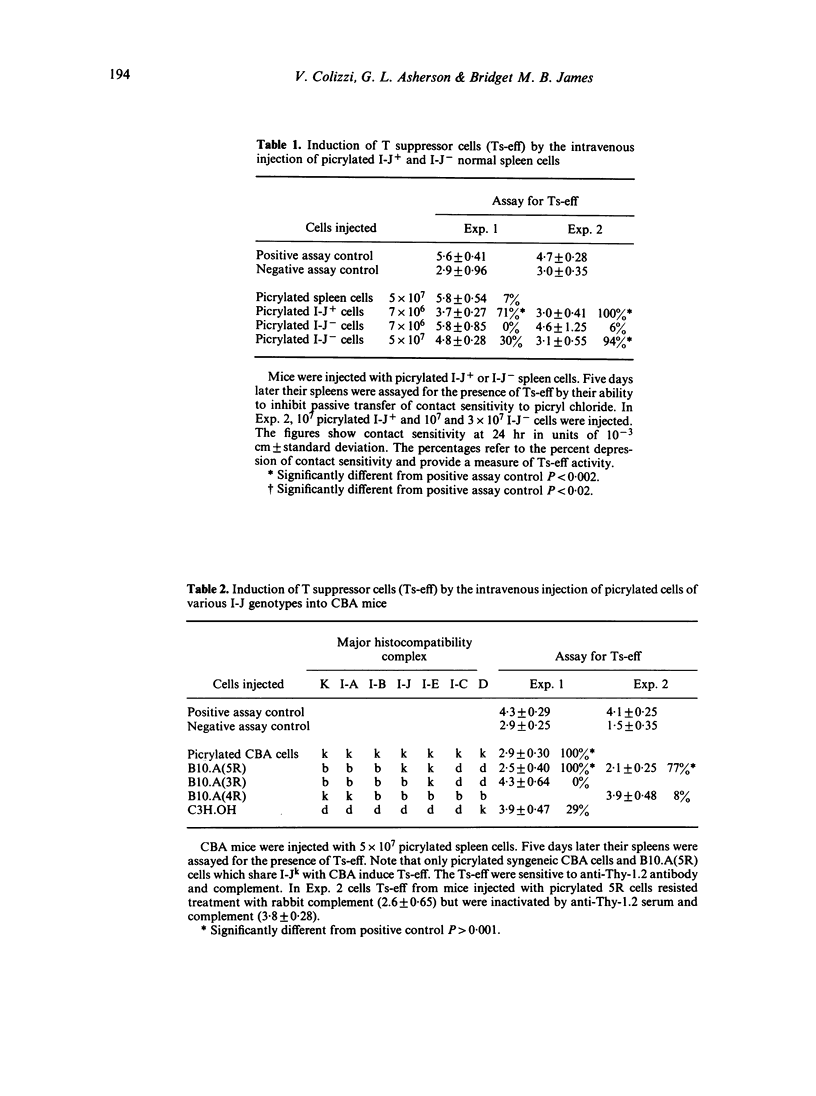
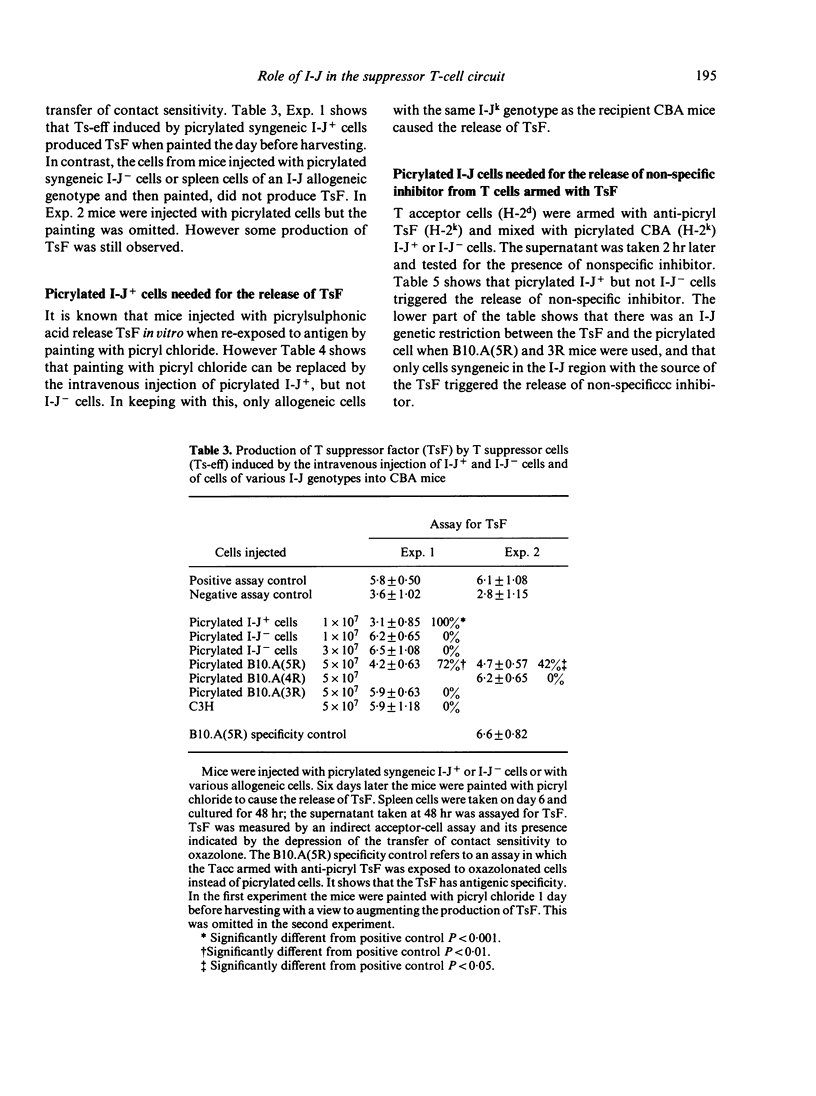
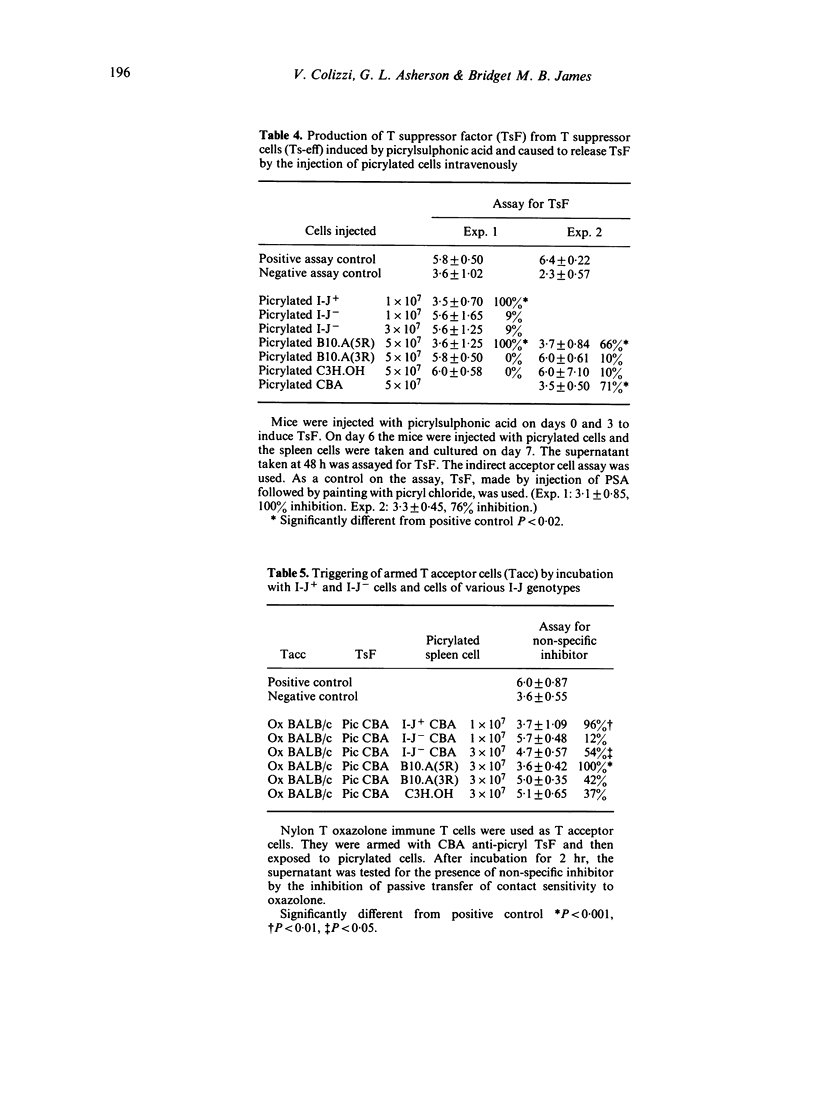
One of the T suppressor circuits induced by picrylsulphonic acid includes the T suppressor cell (Ts-eff) which acts at the efferent stage of the contact sensitivity reaction and produces antigen-specific T suppressor factor (TsF). This factor does not act directly but arms a T acceptor cell (Tacc). This Tacc liberates a non-specific inhibitor when it is armed with TsF and then exposed to picrylated cells sharing the I-J genotype of the source of the TsF. This paper investigates the role of I-J region gene products in this T suppressor circuit. Two approaches were used. Syngeneic CBA (H-2k) lymphocytes were separated into I-J+ and I-J- cells by treatment with anti-I-Jk serum followed by panning on anti-immunoglobulin plates. The cells were then picrylated and used as a source of antigen. Alternatively, B10.A congeneic mice syngeneic (5R) or allogeneic (3R) with CBA at the I-J locus were picrylated and used similarly. The main findings were as follows. (i) The intravenous injection of picrylated I-J+ spleen cells but not a similar number of I-J- cells induced Ts-eff which blocked the transfer of contact sensitivity. Picrylated unseparated cells syngeneic, but not allogeneic, at the I-J locus were also effective. (ii) It is known that the lymphocytes of mice injected wit picrylsulphonic acid and then re-exposed to antigen by painting with picryl chloride liberate TsF in vitro. The re-exposure to antigen can be replaced by the intravenous injection of picrylated I-J+ cells or by cells syngeneic at the I-J locus the day before harvesting the spleen cells. (iii) The release of non-specific inhibitor by Tacc armed with TsF requires exposure to picrylated I-J+ cells or cells syngeneic at the I-J locus. The requirement for antigen on a cell bearing syngeneic I-J suggests that antigen together with I-J is an activation signal in this T-cell circuit. The simplest explanation is that the receptor of the pristine Ts and of the mature Ts-eff is similar to T suppressor factor.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bromberg J. S., Benacerraf B., Greene M. I. Mechanisms of regulation of cell-mediated immunity. VII. Suppressor T cells induced by suboptimal doses of antigen plus an I-J-specific allogeneic effect. J Exp Med. 1981 Feb 1;153(2):437–449. doi: 10.1084/jem.153.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondz B. D., Karaulov A. V., Chervonsky A. V., Blandova Z. K. Requirement for the location of both appropriate and irrelevant H-2 antigens on the same stimulator cell for unspecific DNA-synthesis inhibition by the H-2-antigen-primed, specific suppressor T cells. Immunogenetics. 1982;15(2):167–176. doi: 10.1007/BF00621949. [DOI] [PubMed] [Google Scholar]
- Eardley D. D., Murphy D. B., Kemp J. D., Shen F. W., Cantor H., Gershon R. K. Ly-1 inducer and Ly-1,2 acceptor T cells in the feedback suppression circuit bear an I-J-subregion controlled determinant. Immunogenetics. 1980;11(6):549–557. doi: 10.1007/BF01567824. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Wigzell H., Binz H. Two different VH gene products make up the T-cell receptors. Scand J Immunol. 1976;5(9):993–1001. doi: 10.1111/j.1365-3083.1976.tb03051.x. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Regulation of delayed-type hypersensitivity. VII. The role of I-J subregion gene products in the inhibition of delayed-type hypersensitivity to major histocompatibility antigens by specific suppressor T cells. Eur J Immunol. 1981 Nov;11(11):883–888. doi: 10.1002/eji.1830111107. [DOI] [PubMed] [Google Scholar]
- Miller S. D., Sy M. S., Claman H. N. Suppressor T cell mechanisms in contact sensitivity. I. Efferent blockade by syninduced suppressor T cells. J Immunol. 1978 Jul;121(1):265–273. [PubMed] [Google Scholar]
- Mitchison N. A. Allospecific T cells. Cell Immunol. 1981 Aug;62(2):258–263. doi: 10.1016/0008-8749(81)90324-5. [DOI] [PubMed] [Google Scholar]
- Okuda K., Minami M., Furusawa M., Dorf M. E. Analysis of T cell hybridomas. II. Comparisons among three distinct types of monoclonal suppressor factors. J Exp Med. 1981 Dec 1;154(6):1838–1851. doi: 10.1084/jem.154.6.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Minami M., Sherr D. H., Dorf M. E. Hapten-specific T cell responses to 4-hydroxy-3-nitrophenyl acetyl. XI. Pseudogenetic restrictions of hybridoma suppressor factors. J Exp Med. 1981 Aug 1;154(2):468–479. doi: 10.1084/jem.154.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres A., Bromberg J. S., Sy M. S., Benacerraf B., Greene M. I. Mechanisms of regulation of cell-mediated immunity. VI. Antigen density dependence of the induction of genetically restricted suppressor cells. J Immunol. 1980 Jan;124(1):343–348. [PubMed] [Google Scholar]
- Streilein J. W., Gruchalla R. S. Analysis of neonatally induced tolerance of H-2 alloantigens. I. Adoptive transfer indicates that tolerance of class I and class II antigens is maintained by distinct mechanisms. Immunogenetics. 1981;12(1-2):161–173. doi: 10.1007/BF01561659. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., Klein J. Neonatal tolerance of H-2 alloantigens. I. I region modulation of tolerance potential of K and D antigens. Proc R Soc Lond B Biol Sci. 1980 Apr 22;207(1169):461–474. doi: 10.1098/rspb.1980.0034. [DOI] [PubMed] [Google Scholar]
- Zembala M. A., Asherson G. L., James B. M., Stein V. E., Watkins M. C. Anti-haptene T suppressor factor acts through an I-J+, Ly1-2+, T acceptor cell that releases a nonspecific inhibitor of the transfer of contact sensitivity when exposed to antigen. J Immunol. 1982 Nov;129(5):1823–1829. [PubMed] [Google Scholar]
- Zembala M., Asherson G. L., Colizzi V. Hapten-specific T suppressor factor recognizes both hapten and I-J region products on haptenized spleen cells. Nature. 1982 Jun 3;297(5865):411–413. doi: 10.1038/297411a0. [DOI] [PubMed] [Google Scholar]


