Abstract
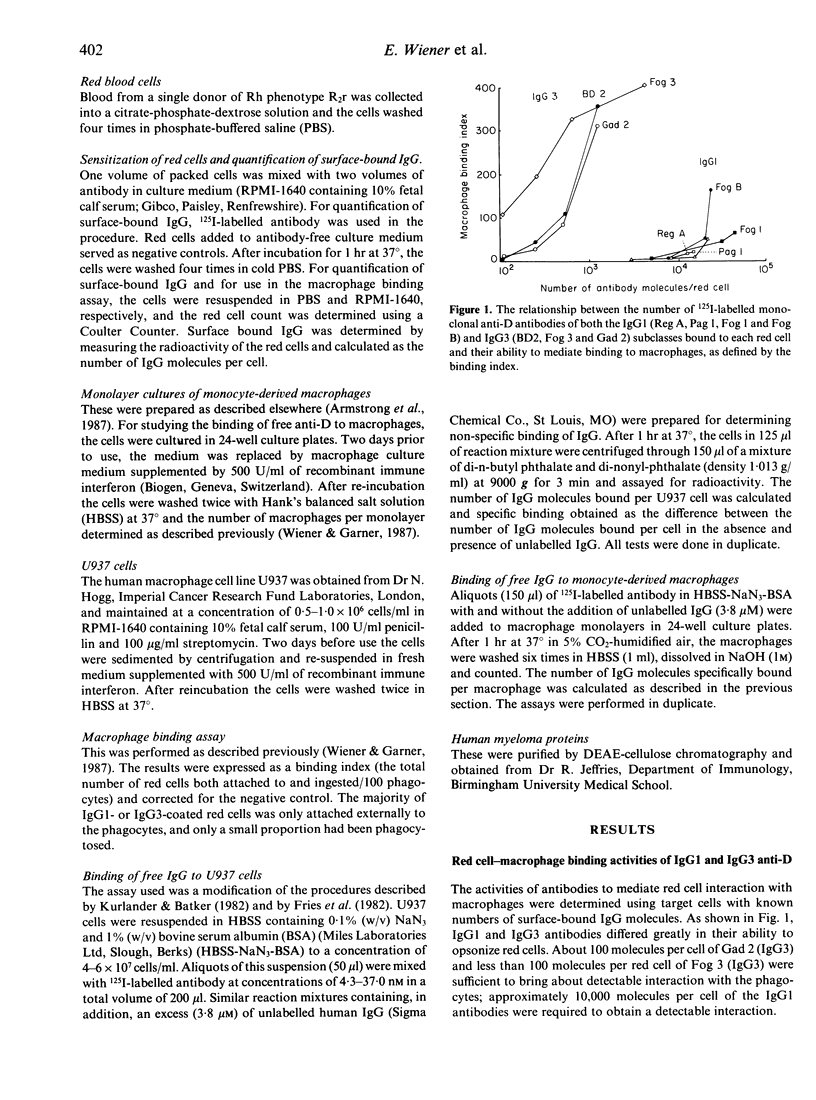
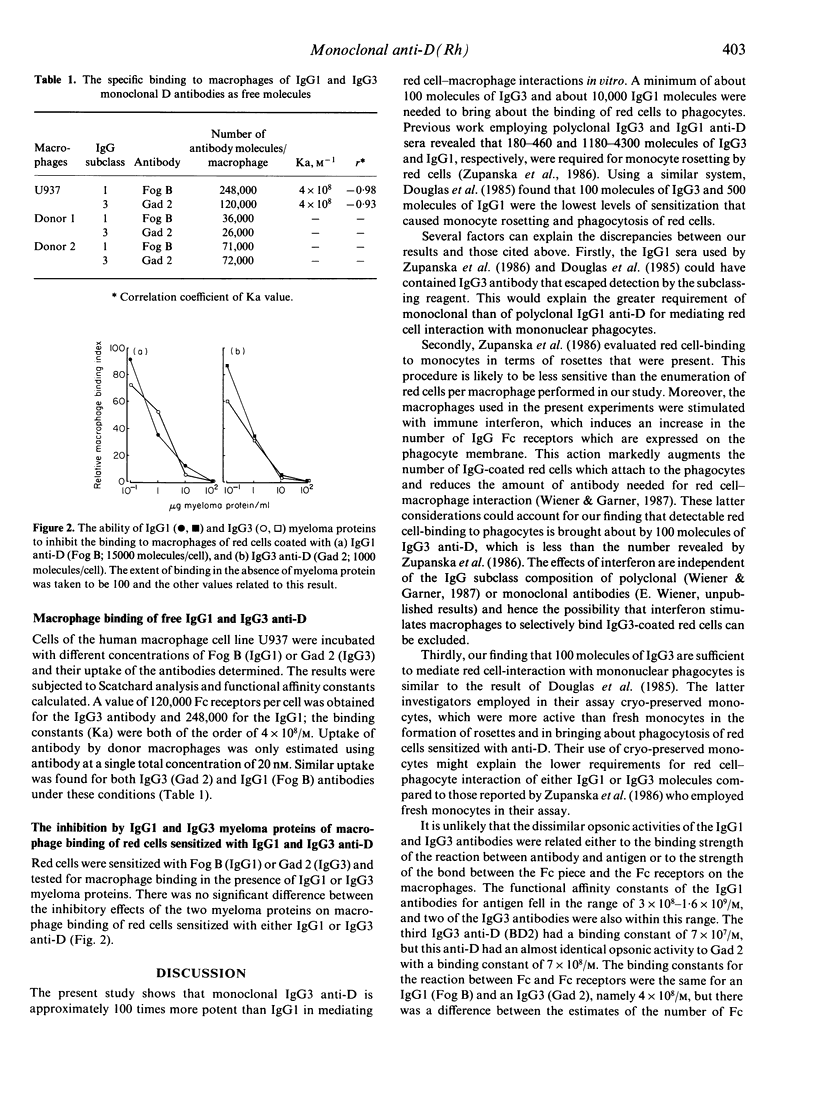
Four IgG1 and three IgG3 human monoclonal antibodies specific for the blood group D(Rh) antigen were tested for their ability to mediate red cell-binding to macrophages in vitro. The IgG3 monoclonals were found to opsonize at a density of approximately 100 molecules per red cell, whereas the IgG1 antibodies were only active at a level of 10,000 molecules per cell. There was no substantial difference between the two IgG subclasses in their ability to bind to Fc receptors on macrophages and it is suggested that the more potent opsonic activity of IgG3 is the result of the relatively long hinge, leading to greater accessibility to the Fc receptor binding site on the Fc piece.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Gelfand E. W., Jandl J. H., Rosen F. S. The interaction between human monocytes and red cells. Specificity for IgG subclasses and IgG fragments. J Exp Med. 1970 Dec 1;132(6):1207–1215. doi: 10.1084/jem.132.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M. D., Andrews J. A., Leslie R. G., Wood N. J. The binding of human and guinea-pig IgG subclasses to homologous macrophage and monocyte Fc receptors. Immunology. 1978 Jul;35(1):115–123. [PMC free article] [PubMed] [Google Scholar]
- Anderson C. L., Abraham G. N. Characterization of the Fc receptor for IgG on a human macrophage cell line, U937. J Immunol. 1980 Dec;125(6):2735–2741. [PubMed] [Google Scholar]
- Douglas R., Rowthorne N. V., Schneider J. V. Some quantitative aspects of the human monocyte erythrophagocytosis and rosette assays. Transfusion. 1985 Nov-Dec;25(6):535–539. doi: 10.1046/j.1537-2995.1985.25686071425.x. [DOI] [PubMed] [Google Scholar]
- Fries L. F., Hall R. P., Lawley T. J., Crabtree G. R., Frank M. M. Monocyte receptors for the Fc portion of IgG studies with monomeric human IgG1: normal in vitro expression of Fc gamma receptors in HLA-B8/Drw3 subjects with defective Fc gamma-mediated in vivo clearance. J Immunol. 1982 Sep;129(3):1041–1049. [PubMed] [Google Scholar]
- Gunson H. H., Phillips P. K., Stratton F. Manipulative and inherent errors in anti-D quantitation using the AutoAnalyzer. J Clin Pathol. 1972 Mar;25(3):198–205. doi: 10.1136/jcp.25.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay F. C., Torrigiani G., Roitt I. M. The binding of human IgG subclasses to human monocytes. Eur J Immunol. 1972 Jun;2(3):257–261. doi: 10.1002/eji.1830020312. [DOI] [PubMed] [Google Scholar]
- Heusser C., Boesman M., Nordin J. H., Isliker H. Effect of chemical and enzymatic radioiodination on in vitro human Clq activities. J Immunol. 1973 Mar;110(3):820–828. [PubMed] [Google Scholar]
- Huber H., Douglas S. D., Nusbacher J., Kochwa S., Rosenfield R. E. IgG subclass specificity of human monocyte receptor sites. Nature. 1971 Feb 5;229(5284):419–420. doi: 10.1038/229419a0. [DOI] [PubMed] [Google Scholar]
- Kurlander R. J., Batker J. The binding of human immunoglobulin G1 monomer and small, covalently cross-linked polymers of immunoglobulin G1 to human peripheral blood monocytes and polymorphonuclear leukocytes. J Clin Invest. 1982 Jan;69(1):1–8. doi: 10.1172/JCI110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsen T. E., Frangione B., Franklin E. C. Primary structure of the "hinge" region of human IgG3. Probable quadruplication of a 15-amino acid residue basic unit. J Biol Chem. 1977 Feb 10;252(3):883–889. [PubMed] [Google Scholar]
- Rochna E., Hughes-Jones N. C. The use of purified 125-I-labelled anti-gamma globulin in the determination of the number of D antigen sites on red cells of different phenotypes. Vox Sang. 1965 Nov-Dec;10(6):675–686. doi: 10.1111/j.1423-0410.1965.tb05179.x. [DOI] [PubMed] [Google Scholar]
- Thompson K. M., Hough D. W., Maddison P. J., Melamed M. D., Hughes-Jones N. The efficient production of stable, human monoclonal antibody-secreting hybridomas from EBV-transformed lymphocytes using the mouse myeloma X63-Ag8.653 as a fusion partner. J Immunol Methods. 1986 Nov 20;94(1-2):7–12. doi: 10.1016/0022-1759(86)90208-5. [DOI] [PubMed] [Google Scholar]
- Thompson K. M., Melamed M. D., Eagle K., Gorick B. D., Gibson T., Holburn A. M., Hughes-Jones N. C. Production of human monoclonal IgG and IgM antibodies with anti-D (rhesus) specificity using heterohybridomas. Immunology. 1986 May;58(1):157–160. [PMC free article] [PubMed] [Google Scholar]
- Woof J. M., Partridge L. J., Jefferis R., Burton D. R. Localisation of the monocyte-binding region on human immunoglobulin G. Mol Immunol. 1986 Mar;23(3):319–330. doi: 10.1016/0161-5890(86)90059-3. [DOI] [PubMed] [Google Scholar]
- Zupańska B., Brojer E., Maślanka K., Hallberg T. A comparison between Fc receptors for IgG1 and IgG3 on human monocytes and lymphocytes using anti-Rh antibodies. Vox Sang. 1985;49(1):67–76. doi: 10.1111/j.1423-0410.1985.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Zupańska B., Thomson E. E., Merry A. H. Fc receptors for IgG1 and IgG3 on human mononuclear cells--an evaluation with known levels of erythrocyte-bound IgG. Vox Sang. 1986;50(2):97–103. doi: 10.1111/j.1423-0410.1986.tb04854.x. [DOI] [PubMed] [Google Scholar]
- van der Meulen F. W., van der Hart M., Fleer A., von dem Borne A. E., Engelfriet C. P., van Loghem J. J. The role of adherence to human mononuclear phagocytes in the destruction of red cells sensitized with non-complement binding IgG antibodies. Br J Haematol. 1978 Apr;38(4):541–549. doi: 10.1111/j.1365-2141.1978.tb01079.x. [DOI] [PubMed] [Google Scholar]


