Abstract
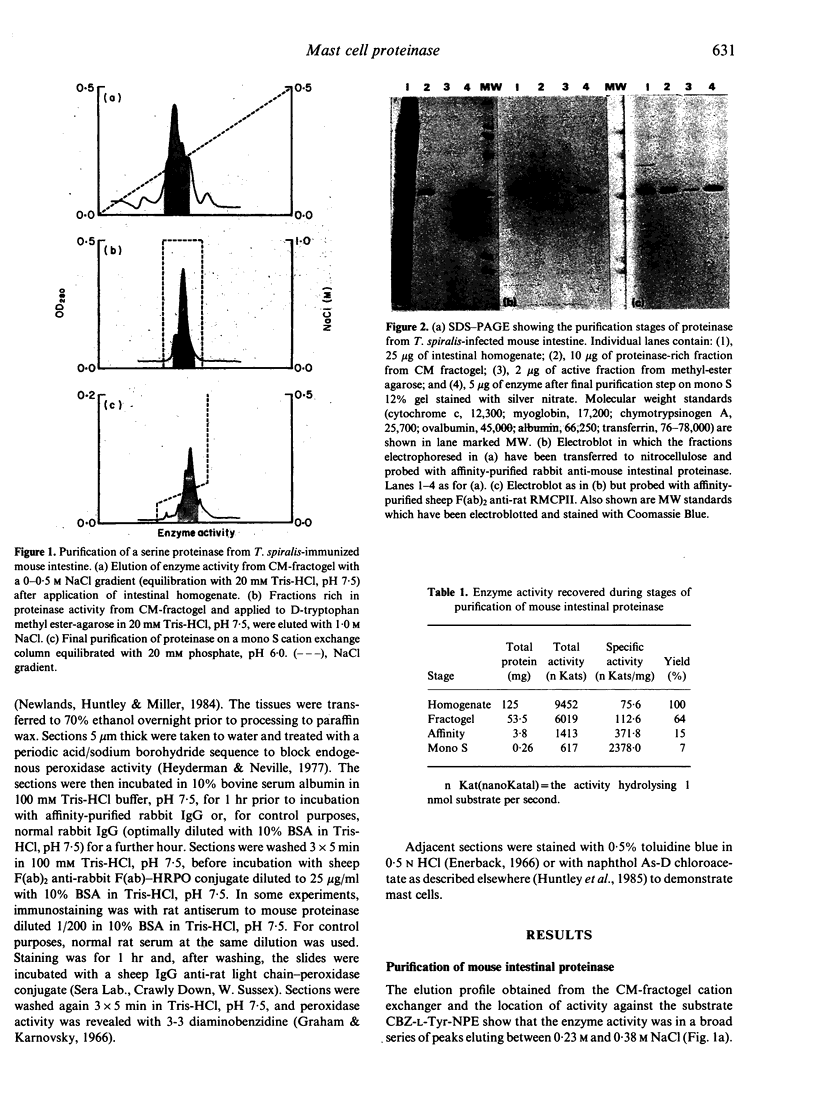
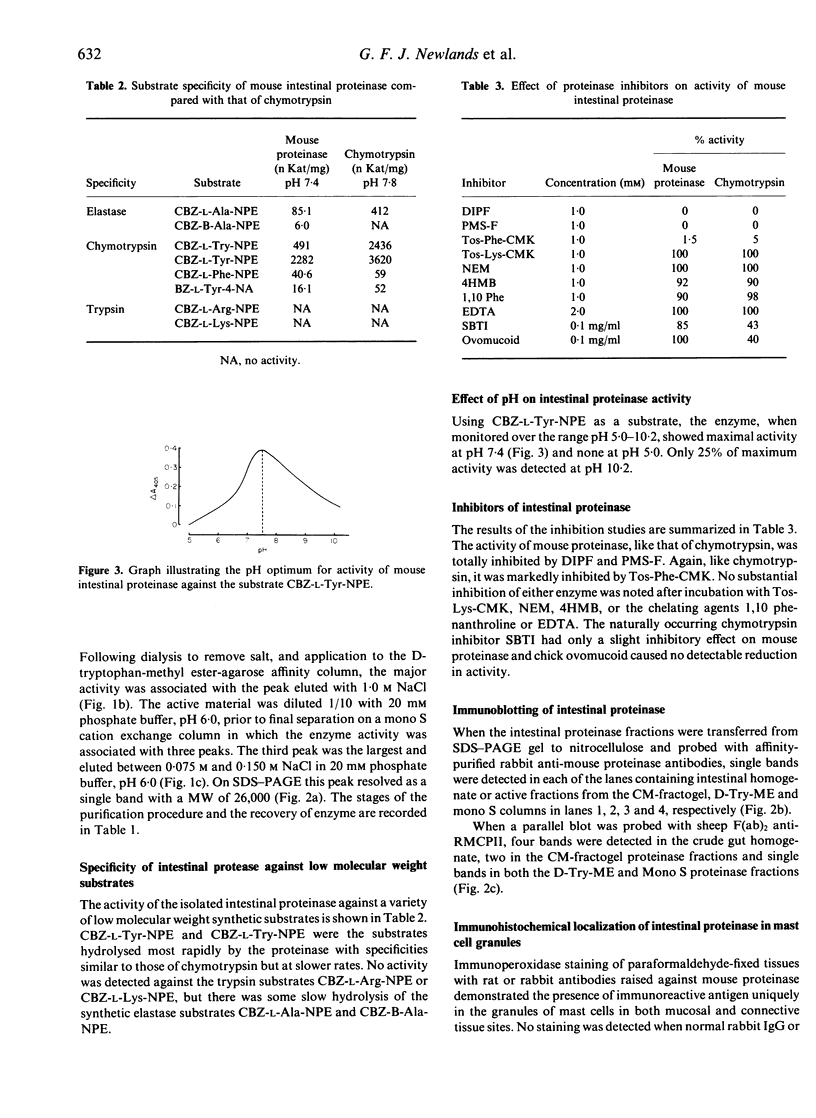
A proteinase was purified by cation exchange and affinity chromatography from the small intestines of mice infected with Trichinella spiralis. The enzyme was highly soluble and was chymotrypsin-like in its substrate specificities and susceptibility to inhibitors. It had a MW of 26,000, as determined by SDS-PAGE electrophoresis. Antibodies raised against the proteinase were affinity purified and their specificity confirmed by Western blot analysis. When used to localize the enzyme immunohistochemically, they reacted with granules of mast cells in the epithelium and lamina propria of the parasitized small intestine. The antibodies also bound to mast cell granules in a number of other sites, including tracheal epithelium, gastric mucosa, skin and tongue. Affinity-purified antibodies raised against rat mast cell proteinase II (RMCPII) cross-reacted with the mouse mast cell proteinase on Western blots.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizadeh H., Wakelin D. Comparison of rapid expulsion of Trichinella spiralis in mice and rats. Int J Parasitol. 1982 Feb;12(1):65–73. doi: 10.1016/0020-7519(82)90097-2. [DOI] [PubMed] [Google Scholar]
- Crowle P. K., Phillips D. E. Characteristics of mast cells in Chediak-Higashi mice: light and electron microscopic studies of connective tissue and mucosal mast cells. Exp Cell Biol. 1983;51(3):130–139. doi: 10.1159/000163183. [DOI] [PubMed] [Google Scholar]
- DuBuske L., Austen K. F., Czop J., Stevens R. L. Granule-associated serine neutral proteases of the mouse bone marrow-derived mast cell that degrade fibronectin: their increase after sodium butyrate treatment of the cells. J Immunol. 1984 Sep;133(3):1535–1541. [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66(3):303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Gibson S., Miller H. R. Mast cell subsets in the rat distinguished immunohistochemically by their content of serine proteinases. Immunology. 1986 May;58(1):101–104. [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Dy M., Luffau G., Vassalli P. Gut mucosal mast cells. Origin, traffic, and differentiation. J Exp Med. 1984 Jul 1;160(1):12–28. doi: 10.1084/jem.160.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. M., McKee T. A., Jarrett E. E., Woodbury R., Miller H. R. Generation of mucosal mast cells is stimulated in vitro by factors derived from T cells of helminth-infected rats. Nature. 1982 Nov 11;300(5888):188–190. doi: 10.1038/300188a0. [DOI] [PubMed] [Google Scholar]
- Heyderman E., Neville A. M. A shorter immunoperoxidase technique for the demonstration of carcinoembryonic antigen and other cell products. J Clin Pathol. 1977 Feb;30(2):138–140. doi: 10.1136/jcp.30.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley J. F., Newlands G. F., Gibson S., Ferguson A., Miller H. R. Histochemical demonstration of chymotrypsin like serine esterases in mucosal mast cells in four species including man. J Clin Pathol. 1985 Apr;38(4):375–384. doi: 10.1136/jcp.38.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani A. A., Schechter N. M., Craig S. S., DeBlois G., Schwartz L. B. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D. P., Gibson S., Huntley J. F. The catalytic properties of a proteinase isolated from sheep abomasal mucosal mast cells. Int J Biochem. 1986;18(10):961–964. doi: 10.1016/0020-711x(86)90079-0. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Woodbury R. G., Huntley J. F., Newlands G. Systemic release of mucosal mast-cell protease in primed rats challenged with Nippostrongylus brasiliensis. Immunology. 1983 Jul;49(3):471–479. [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Newlands G. F., Huntley J. F., Miller H. R. Concomitant detection of mucosal mast cells and eosinophils in the intestines of normal and Nippostrongylus-immune rats. A re-evaluation of histochemical and immunocytochemical techniques. Histochemistry. 1984;81(6):585–589. doi: 10.1007/BF00489539. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976 Nov 18;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Sredni B., Friedman M. M., Bland C. E., Metcalfe D. D. Ultrastructural, biochemical, and functional characteristics of histamine-containing cells cloned from mouse bone marrow: tentative identification as mucosal mast cells. J Immunol. 1983 Aug;131(2):915–922. [PubMed] [Google Scholar]
- Vensel W. H., Komender J., Barnard E. A. Non-pancreatic proteases of the chymotrypsin family. II. Two proteases from a mouse mast cell tumor. Biochim Biophys Acta. 1971 Nov 13;250(2):395–407. doi: 10.1016/0005-2744(71)90196-3. [DOI] [PubMed] [Google Scholar]
- Wenzel S., Irani A. M., Sanders J. M., Bradford T. R., Schwartz L. B. Immunoassay of tryptase from human mast cells. J Immunol Methods. 1986 Jan 22;86(1):139–142. doi: 10.1016/0022-1759(86)90277-2. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Gruzenski G. M., Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2785–2789. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Neurath H. Purification of an atypical mast cell protease and its levels in developing rats. Biochemistry. 1978 Oct 3;17(20):4298–4304. doi: 10.1021/bi00613a029. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Neurath H. Structure, specificity and localization of the serine proteases of connective tissue. FEBS Lett. 1980 Jun 2;114(2):189–196. doi: 10.1016/0014-5793(80)81112-4. [DOI] [PubMed] [Google Scholar]