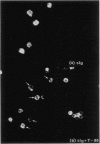
Abstract
The results presented in this paper demonstrate that a mouse IgM monoclonal antibody (T-80) recognizes an antigen on cells of the T-lymphocyte lineage of sheep. However, this antibody does not identify all T cells, as 10-20% of thymocytes and some peripheral-blood T cells are negative. T-80- thymocytes reside in the medulla. The majority of cortical thymocytes are T-80+ and classified as dull cells on the basis of antigen density per cell as measured by flow microfluorometry. In contrast, T-80+ cells in the periphery can be categorized into two populations, i.e., dull cells and bright cells. Suggestive evidence was obtained that bright T-80+ cells are fast recirculating T cells, whereas dull cells are sessile or less easily mobilizable T cells in the periphery. In foetal environment, over 90% of thymocytes and approximately 5% of spleen cells are T-80+ at 54 days of gestation (gestation period = 150 days), which may indicate that T-cell emigration from the thymus commences well before mid-gestation in sheep.
Full text
PDF


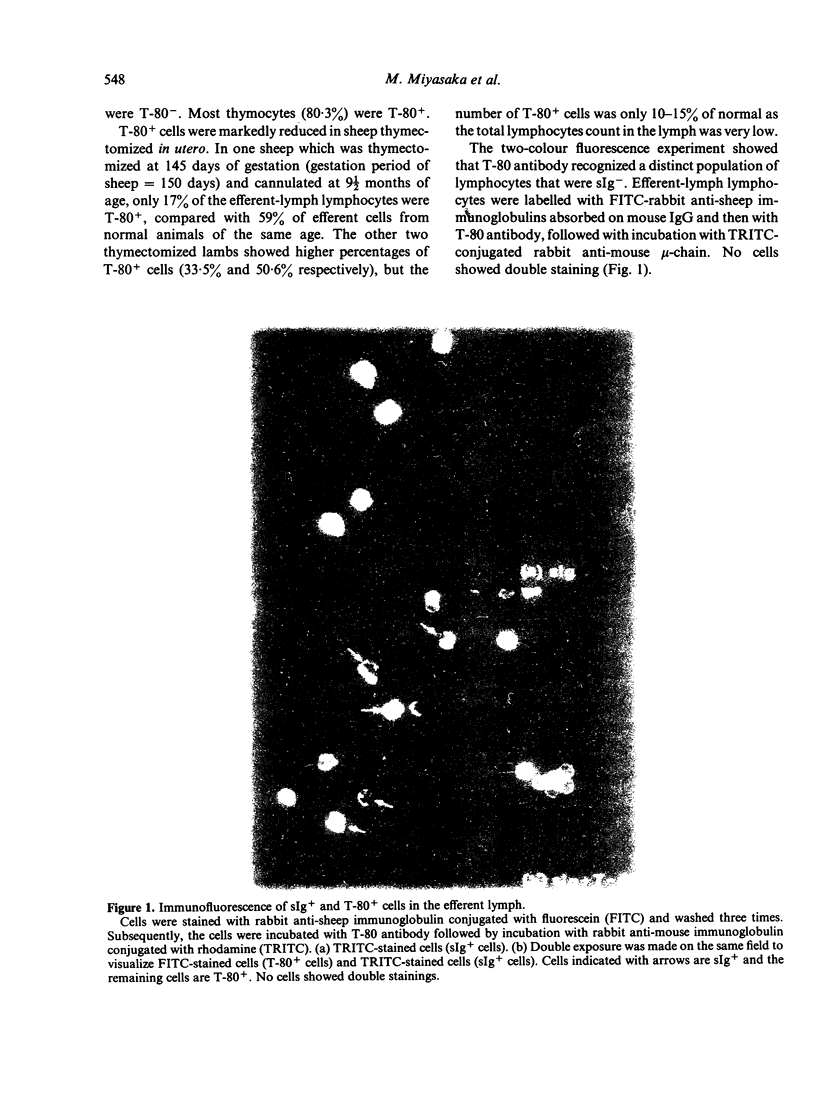
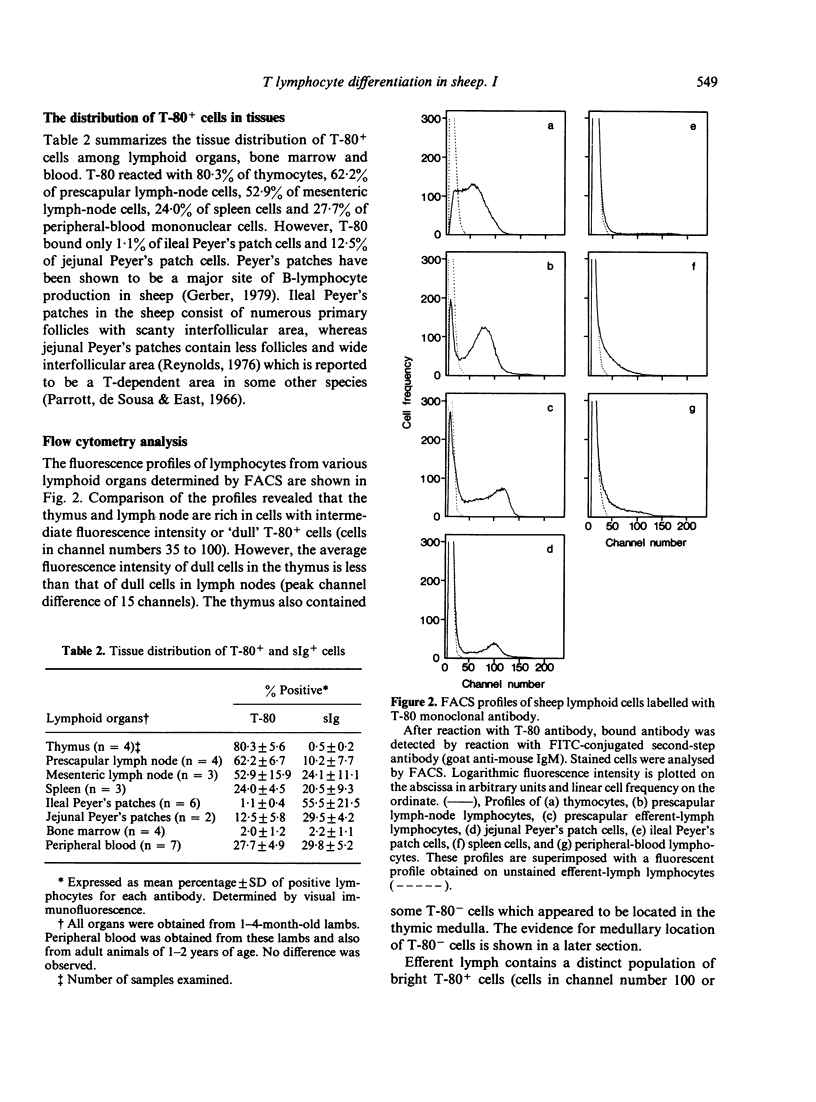
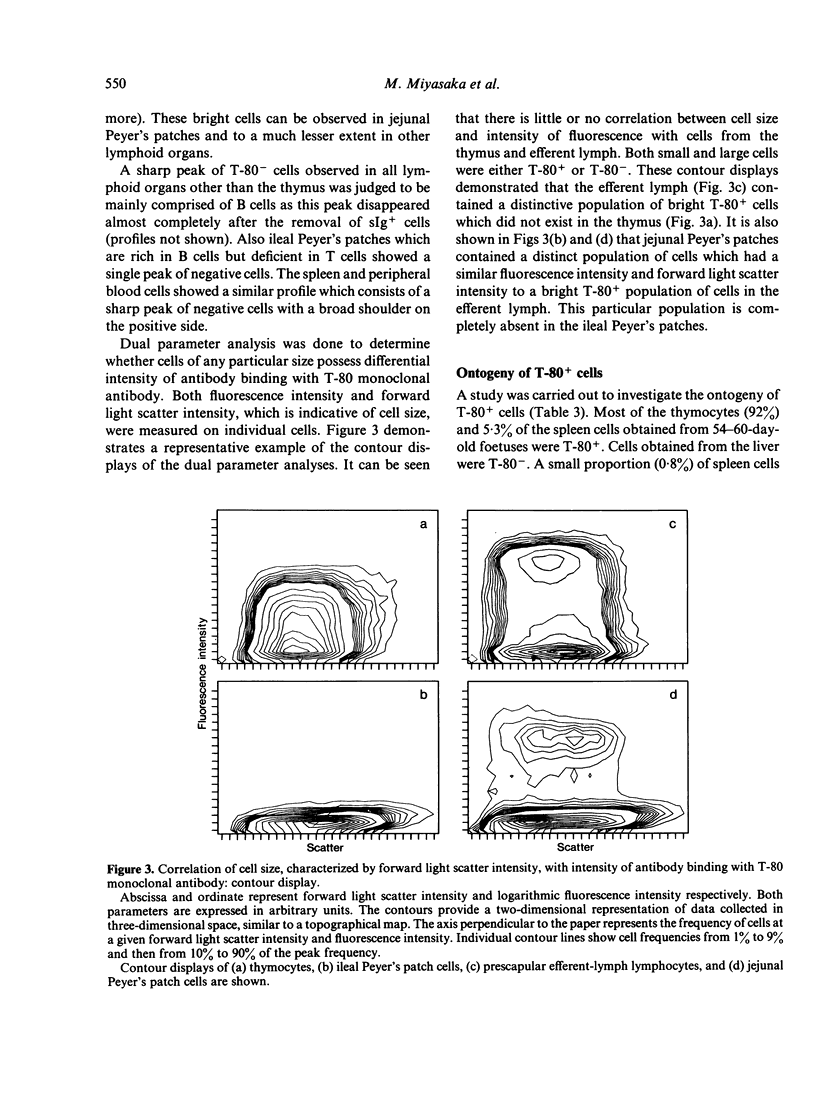



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basch R. S., Berman J. W. Thy-1 determinants are present on many murine hematopoietic cells other than T cells. Eur J Immunol. 1982 May;12(5):359–364. doi: 10.1002/eji.1830120502. [DOI] [PubMed] [Google Scholar]
- Cahill R. N., Poskitt D. C., Frost H., Julius M. H., Trnka Z. Behaviour of sheep-immunoglobulin-bearing and non-immunoglobulin-bearing lymphocytes isolated by nylon wool columns. Int Arch Allergy Appl Immunol. 1978;57(1):90–96. doi: 10.1159/000232088. [DOI] [PubMed] [Google Scholar]
- Cahill R. N., Poskitt D. C., Heron I., Trnka Z. Collection of lymph from single lymph nodes and the intestines of fetal lambs in utero. Int Arch Allergy Appl Immunol. 1979;59(1):117–120. doi: 10.1159/000232248. [DOI] [PubMed] [Google Scholar]
- Cole G. J., Morris B. Homograft rejection and hypersensitivity reactions in lambs thymectomized in utero. Aust J Exp Biol Med Sci. 1971 Feb;49(1):75–88. doi: 10.1038/icb.1971.6. [DOI] [PubMed] [Google Scholar]
- Cole G. J., Morris B. The cellular and humoral response to antigens in lambs thymectomized in utero. Aust J Exp Biol Med Sci. 1971 Feb;49(1):55–73. doi: 10.1038/icb.1971.5. [DOI] [PubMed] [Google Scholar]
- Cole G. J., Morris B. The growth and development of lambs thymectomized in utero. Aust J Exp Biol Med Sci. 1971 Feb;49(1):33–53. doi: 10.1038/icb.1971.4. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- Fahey K. J. The binding of lectins to sheep tissues and circulating cells: peanut agglutinin, a marker for presumptive T-lymphocytes. Aust J Exp Biol Med Sci. 1980 Dec;58(6):557–569. doi: 10.1038/icb.1980.57. [DOI] [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- Garson J. A., Beverley P. C., Coakham H. B., Harper E. I. Monoclonal antibodies against human T lymphocytes label Purkinje neurones of many species. Nature. 1982 Jul 22;298(5872):375–377. doi: 10.1038/298375a0. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Metcalf D., Mandel T., Bollum F. J. Analysis of rat hemopoietic cells on the fluorescence-activated cell sorter. II. Isolation of terminal deoxynucleotidyl transferase-positive cells. J Exp Med. 1980 Aug 1;152(2):438–446. doi: 10.1084/jem.152.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Miyasaka M., McCullagh P. Immunological responsiveness of maternal and foetal lymphocytes during normal pregnancy in the ewe. J Reprod Immunol. 1981 May;3(1):15–27. doi: 10.1016/0165-0378(81)90025-5. [DOI] [PubMed] [Google Scholar]
- Miyasaka M., McCullagh P. The response of the foetal lamb to maternal lymphocytes. J Reprod Immunol. 1982 Aug;4(4):217–230. doi: 10.1016/0165-0378(82)90028-6. [DOI] [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Sunderland C. A., Allington M. J., Stein H., Mason D. Y. The value of immunohistological screening in the production of monoclonal antibodies. J Immunol Methods. 1982;50(2):145–160. doi: 10.1016/0022-1759(82)90221-6. [DOI] [PubMed] [Google Scholar]
- Outteridge P. M., Fahey K. J., Lee C. S. Lymphocyte surface markers in sheep blood and lymph. Aust J Exp Biol Med Sci. 1981 Apr;59(Pt 2):143–155. doi: 10.1038/icb.1981.10. [DOI] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L. D., Simpson-Morgan M. W., Morris B. Lymphopoiesis and lymphocyte recirculation in the sheep fetus. J Exp Med. 1976 Jan 1;143(1):167–186. doi: 10.1084/jem.143.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. C., Morris B. The role of the lymphatic system in the rejection of homografts: a study of lymph from renal transplants. J Exp Med. 1970 May 1;131(5):936–969. doi: 10.1084/jem.131.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- SCHINCKEL P. G., FERGUSON K. A. Skin transplantation in the foetal lamb. Aust J Biol Sci. 1953 Aug;6(3):533–546. doi: 10.1071/bi9530533. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN A. M., PRENDERGAST R. A., KRANER K. L. FETAL RESPONSE TO ANTIGENIC STIMULUS. IV. REJECTION OF SKIN HOMOGRAFTS BY THE FETAL LAMB. J Exp Med. 1964 Jan 1;119:955–964. doi: 10.1084/jem.119.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Battye F., Scollay R. Expression of Thy-1 antigen is not limited to T cells in cultures of mouse hemopoietic cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4161–4165. doi: 10.1073/pnas.79.13.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L. Significance of Lyt phenotypes: Lyt2 antibodies block activities of T cells that recognize class 1 major histocompatibility complex antigens regardless of their function. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7101–7105. doi: 10.1073/pnas.78.11.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele H. G., Stark R., Keeser D. Antigenic correlations between brain and thymus. I. Common antigenic structures in rat and mouse brain tissue and thymocytes. Eur J Immunol. 1972 Oct;2(5):424–429. doi: 10.1002/eji.1830020508. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]