Abstract
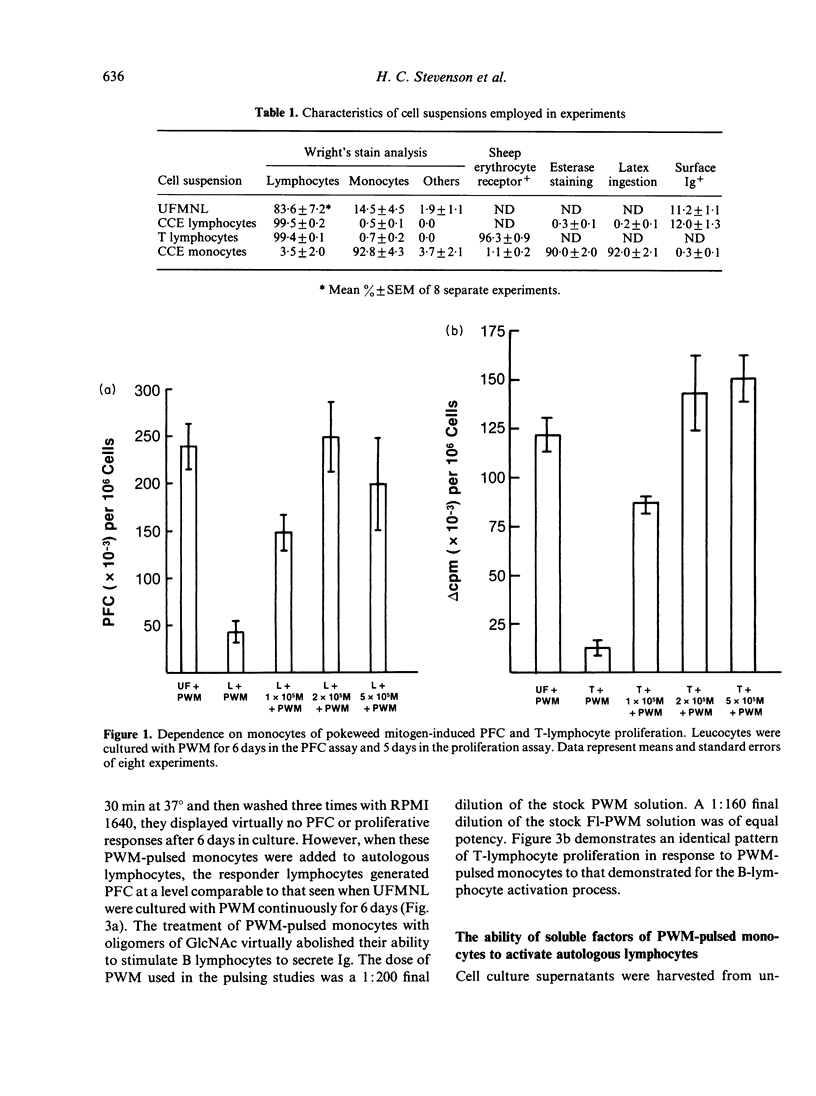
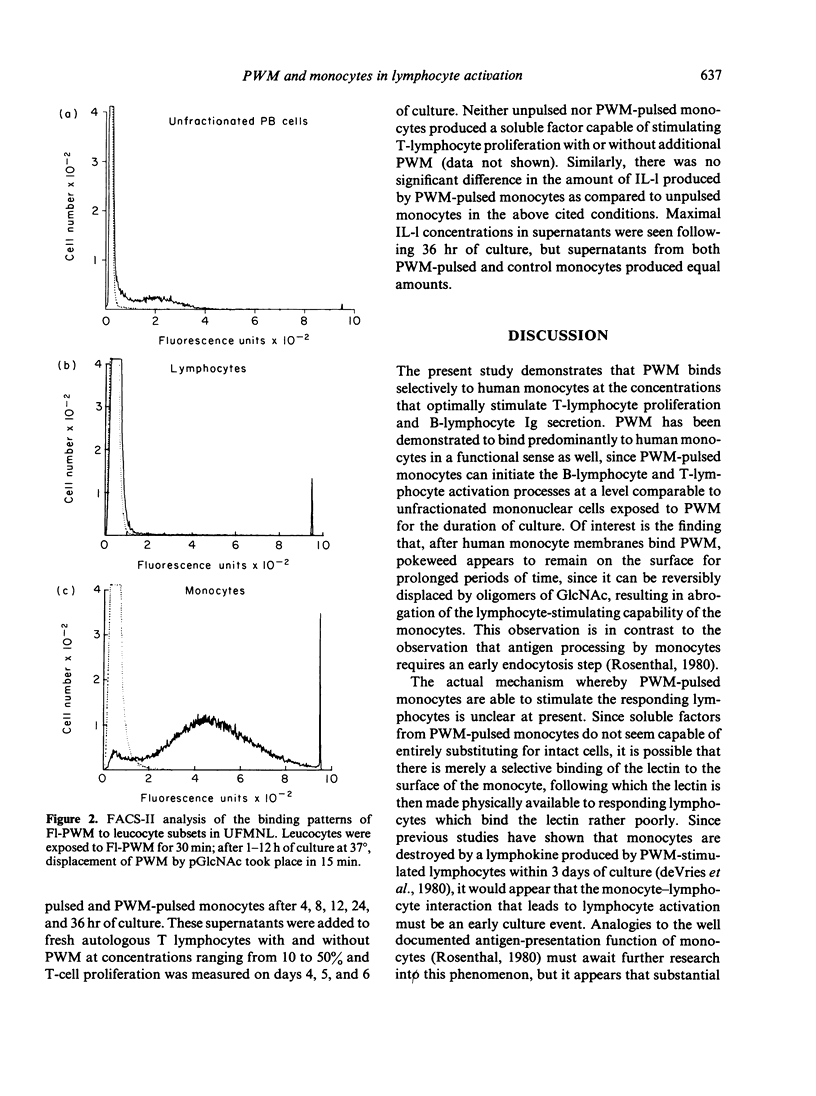
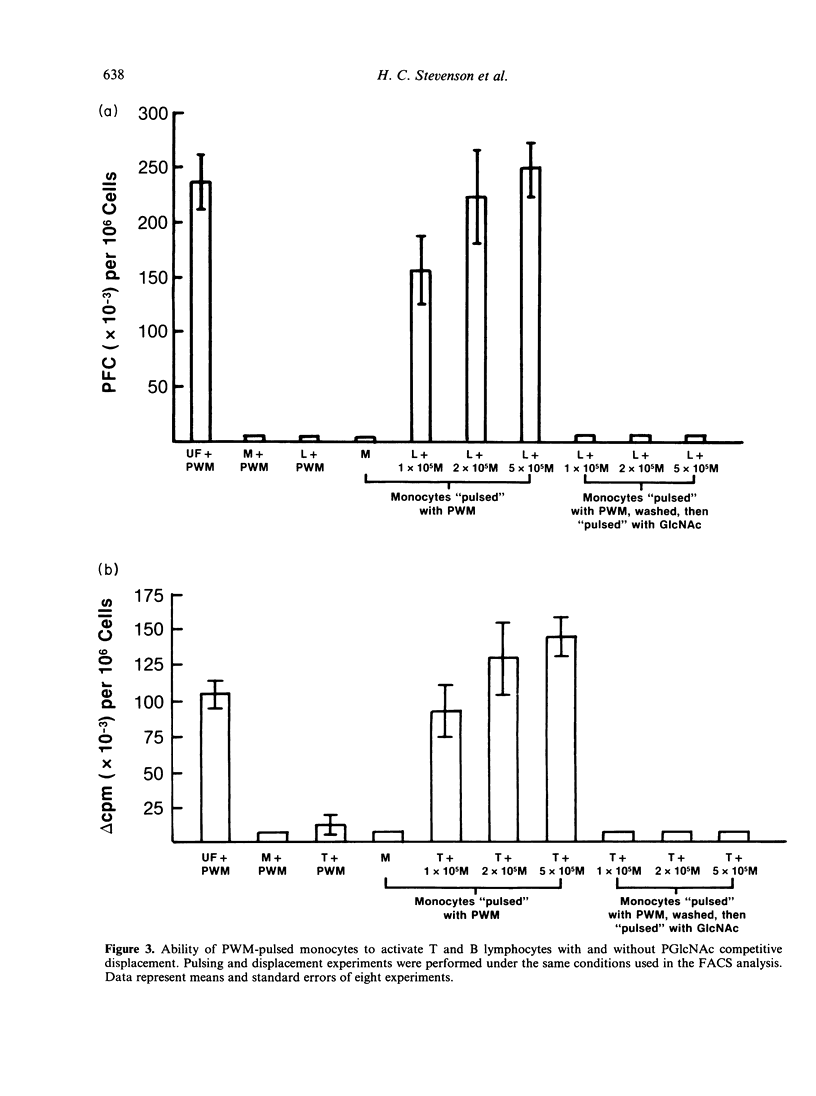
The present study examines the role of monocytes in the in-vitro activation of human T cells and B cells by pokeweed mitogen (PWM). The T cell-dependent PWM-induced B-cell activation process was found to be monocyte dependent. Fluorescence-activated cell sorter (FACS) analysis revealed that upon addition to peripheral blood mononuclear cells, fluoresceinated PWM, at concentrations that provided optimal B-cell and T-cell activation, bound predominantly to human monocytes. The binding of PWM to monocytes was reversible and could be displaced within the first few hours of binding by oligomers of N-acetylglucosamine (GlcNAc). As a functional correlate of the binding studies, it was shown that PWM-pulsed monocytes could induce B lymphocytes to become plaque-forming cells (PFC) and T lymphocytes to undergo proliferation. In contrast, markedly reduced PFC and blastogenic responses were observed when monocyte-depleted B lymphocytes and T lymphocytes were respectively pulsed with PWM and washed, followed by the addition of non-PWM-pulsed monocytes to the cultures. Thus, the initial event in the PWM-induced activation of human lymphocytes, for both in-vitro T-lymphocyte blastogenic responses and B-lymphocyte Ig secretion, appears to be binding of the mitogen to sugar residues on the surface membrane of the monocyte, followed by subsequent interaction with the appropriate lymphocytes. The process of PWM binding to monocytes did not appear to affect the baseline production of interleukin-1 (IL-1) by human monocytes, nor could soluble factors from PWM-pulsed monocytes substitute for intact cells in the initiation of the lymphocyte-activation process.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Contreras T. J., Jemionek J. F., Stevenson H. C., Hartwig V. M., Fauci A. S. An improved technique for the negative selection of large numbers of human lymphocytes and monocytes by counterflow centrifugation--elutriation. Cell Immunol. 1980 Aug 15;54(1):215–229. doi: 10.1016/0008-8749(80)90203-8. [DOI] [PubMed] [Google Scholar]
- Dimitriu A., Fauci A. S. Activation of human B lymphocytes. IX. Modulation of antibody production by products of activated macrophages. J Immunol. 1978 Jun;120(6):1818–1823. [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. Alternate-day prednisone therapy and human lymphocyte subpopulations. J Clin Invest. 1975 Jan;55(1):22–32. doi: 10.1172/JCI107914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Human B cell function in a polyclonally induced plaque forming cell system. Cell triggering and immunoregulation. Immunol Rev. 1979;45:93–116. doi: 10.1111/j.1600-065x.1979.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R., Whalen G. Activation of human B lymphocytes. II. Cellular interactions in the PFC response of human tonsillar and peripheral blood B lymphocytes to polyclonal activation by pokeweed mitogen. J Immunol. 1976 Dec;117(6):2100–2104. [PubMed] [Google Scholar]
- Fauci A. S., Whalen G., Burch C. Activation of human B lymphocytes. XV. Spontaneously occurring and mitogen-induced indirect anti-sheep red blood cell plaque-forming cells in normal human peripheral blood. J Immunol. 1980 May;124(5):2410–2413. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. VI. Immunoregulation of antibody production by mitogen-induced and naturally occurring cells in normal individuals. Cell Immunol. 1978 Mar 15;36(2):294–302. doi: 10.1016/0008-8749(78)90273-3. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. IV. Effects of in vitro hydrocortisone on naturally occuring and mitogen-induced suppressor cells in man. Cell Immunol. 1979 Apr;44(1):157–168. doi: 10.1016/0008-8749(79)90036-4. [DOI] [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Nilsson S. F., Waxdal M. J. Isolation and characterization of lectin binding proteins from murine lymphoid cells. Biochemistry. 1978 Mar 7;17(5):903–910. doi: 10.1021/bi00598a025. [DOI] [PubMed] [Google Scholar]
- Pillai P. S., Scott D. W., Piper M., Corley R. B. Effect of recent antigen exposure on the functional expression of B cell subpopulations. J Immunol. 1982 Sep;129(3):1023–1028. [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol. 1979 Mar;122(3):926–931. [PubMed] [Google Scholar]
- Rosenthal A. S. Regulation of the immune response--role of the macrophage. N Engl J Med. 1980 Nov 13;303(20):1153–1156. doi: 10.1056/NEJM198011133032005. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Stevenson H. C., Fauci A. S. Activation of human B lymphocytes. XII. Differential effects of in vitro cyclophosphamide on human lymphocyte subpopulations involved in B-cell activation. Immunology. 1980 Mar;39(3):391–397. [PMC free article] [PubMed] [Google Scholar]
- Waxdal M. J. Isolation, characterization, and biological activities of five mitogens from pokeweed. Biochemistry. 1974 Aug 27;13(18):3671–3677. doi: 10.1021/bi00715a008. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Terao T., Osawa T. Carbohydrate-binding specificity of pokeweed mitogens. Biochim Biophys Acta. 1978 Jan 18;538(2):384–396. doi: 10.1016/0304-4165(78)90366-5. [DOI] [PubMed] [Google Scholar]
- de Vries E., Lafeber G. J., van der Weij J. P., van Buijsen A. C., Leijh P. C., Cats A. Pokeweed-mitogen induced lymphocyte proliferation: the effect of stimulation on mononuclear phagocytic cells. Immunology. 1980 Jun;40(2):177–182. [PMC free article] [PubMed] [Google Scholar]
- de Vries J. E., Caviles A. P., Jr, Bont W. S., Mendelsohn J. The role of monocytes in human lymphocyte activation by mitogens. J Immunol. 1979 Mar;122(3):1099–1107. [PubMed] [Google Scholar]


