Abstract
Here, we show that the budding yeast proteins Ndc80p, Nuf2p, Spc24p and Spc25p interact at the kinetochore. Consistently, Ndc80p, Nuf2p, Spc24p and Spc25p associate with centromere DNA in chromatin immunoprecipitation experiments, and SPC24 interacts genetically with MCM21 encoding a kinetochore component. Moreover, although conditional lethal spc24-2 and spc25-7 cells form a mitotic spindle, the kinetochores remain in the mother cell body and fail to segregate the chromosomes. Despite this defect in chromosome segregation, spc24-2 and spc25-7 cells do not arrest in metaphase in response to checkpoint control. Furthermore, spc24-2 cells showed a mitotic checkpoint defect when microtubules were depolymerized with nocodazole, indicating that Spc24p has a function in checkpoint control. Since Ndc80p, Nuf2p and Spc24p are conserved proteins, it is likely that similar complexes are part of the kinetochore in other organisms.
Keywords: kinetochore/Ndc80p/Nuf2p/Spc24p/Spc25p
Introduction
The centromere–kinetochore complex ensures high-fidelity chromosome segregation in mitosis and meiosis by mediating the attachment and movement of chromosomes along spindle microtubules (Pidoux and Allshire, 2000). Kinetochores from different organisms vary widely in terms of length. In fission yeast, the centromere DNA is 40–100 kbp (Takahashi et al., 1992) and in mammalian cells up to 1000 kbp long. In contrast, the centromere DNA of budding yeast is a relatively simple assembly of 125 bp (Fitzgerald-Hayes et al., 1982).
A number of budding yeast kinetochore proteins are conserved. Cse4p, a histone H3 variant, is a component of the core centromere of Saccharomyces cerevisiae and shows homology to the human centromere protein CENP-A (Meluh et al., 1998). Mif2p is another conserved component of the yeast kinetochore that displays similarity to mammalian CENP-C (Meluh and Koshland, 1995). Additional budding yeast core kinetochore components are Ndc10p, Cep3p, Ctf13p and Skp1p, which form the CBF3 subcomplex (Lechner and Carbon, 1991; Goh and Kilmartin, 1993; Lechner, 1994; Stemmann and Lechner, 1996). A further subcomplex, composed of Ctf19p, Mcm21p and Okp1p, localizes to yeast centromere DNA in a CBF3-dependent manner (Ortiz et al., 1999). Similarly, centromere association of Mtw1p, a homologue of the fission yeast kinetochore protein Mis12, was dependent on Ndc10p (Goshima and Yanagida, 2000).
In S.cerevisiae, sister centromeres undergo cycles of splitting and rejoining at the end of S-phase, while the chromosome arms remain attached until the metaphase to anaphase transition (Goshima and Yanagida, 2000; He et al., 2000; Tanaka et al., 2000). This separation is probably due to forces exerted at the sister kinetochores after bipolar attachment to the mitotic spindle (Goshima and Yanagida, 2000). As a result of these forces, the cohesin complexes that establish sister chromatid cohesion probably disassemble or are displaced from the kinetochore, while the complexes localized along the chromosome arms remain bound (Tanaka et al., 2000).
The budding yeast kinetochores are clustered throughout the cell cycle near the microtubule organizing centre, known as the spindle pole body (SPB) (Goh and Kilmartin, 1993; Hyland et al., 1999; Goshima and Yanagida, 2000; He et al., 2000; Jin et al., 2000; Tanaka et al., 2000). Measurements of the distance between the yeast γ-tubulin, named Tub4p, which is associated with the nuclear side of the SPB (Spang et al., 1996), and Mtw1p, a centromere component, revealed that kinetochores of fixed cells are on average only ∼0.3–0.4 µm apart from the SPB (Goshima and Yanagida, 2000). Time-lapse experiments using the central SPB component Spc42p (Donaldson and Kilmartin, 1996) as an SPB marker indicated that the distance between the SPB core and the kinetochore fluctuates between 0.5 and 2.5 µm before onset of anaphase B. During anaphase B, the distance between the SPB and the kinetochore is relatively constant, at 0.5–1 µm (He et al., 2000; Tanaka et al., 2000).
Proteins close to the inner plaque of the SPB, which organizes the nuclear microtubules, have been identified upon isolation of SPBs (Wigge et al., 1998). We have analysed some of these proteins and found that Ndc80p (Rout and Kilmartin, 1990), Nuf2p (Osborne et al., 1994), Spc24p and Spc25p (Wigge et al., 1998) interact at the kinetochore. Moreover, our data suggest that at least Spc24p and Spc25p have a role in spindle checkpoint control and in centromere clustering. Interestingly, homologues of Ndc80p, Nuf2p and Spc24p are present in other organisms, raising the possibility that these proteins are also associated with the kinetochore.
Results
Purification of Spc25p reveals complex formation with Ndc80p, Nuf2p and Spc24p
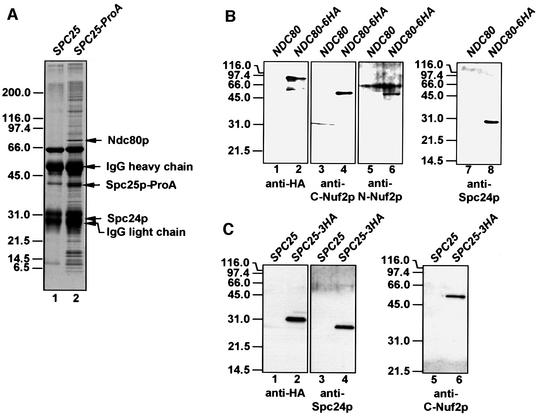
Spc25p is a protein of unknown function that co-purifies with budding yeast SPBs (Wigge et al., 1998). In order to understand the role of Spc25p, a functional, chromosomal gene fusion of SPC25 and protein A (ProA) was constructed. The Spc25p–ProA fusion protein was purified from yeast cell extracts using magnetic beads coated with IgGs, to which ProA binds with high affinity. As a control for proteins that bind non-specifically, an extract from cells without a ProA tag was incubated with the IgG beads. Proteins that were present in the Spc25p–ProA purification (Figure 1A, lane 2) but not in the control (lane 1) were analysed by matrix-assisted laser desorption/ionization (MALDI) analysis (Shevchenko et al., 1996). The strong protein bands of ∼30, 42 and 80 kDa were identified as Spc24p, Spc25p–ProA and Ndc80p. Ndc80p, Spc24p and Spc25p are proteins of unknown function, which are associated with the nuclear side of the SPB (Rout and Kilmartin, 1990; Wigge et al., 1998).
Fig. 1. Ndc80p, Nuf2p, Spc24p and Spc25p are present in common complexes. (A) Purification of Spc25p-associated proteins. Magnetic beads coated with rabbit IgGs were incubated with a yeast extract of wild-type (lane 1) or SPC25–ProA cells (lane 2). Bound proteins were eluted and separated by SDS–PAGE. Coomassie Blue-stained proteins were analysed by MALDI analysis. The major protein bands were identified as Ndc80p, Spc25p–ProA and Spc24p. The minor bands were contaminants like ribosomal proteins. (B) Co-immunoprecipitation of Ndc80p, Nuf2p and Spc24p. Extracts of NDC80 (lanes 1, 3, 5 and 7) or NDC80-6HA cells (lanes 2, 4, 6 and 8) were incubated with anti-HA beads. The precipitate was analysed by immunoblotting using anti-HA (lanes 1 and 2), anti-C-Nuf2p (lanes 3 and 4), anti-N-Nuf2p (lanes 5 and 6) and anti-Spc24p antibodies (lanes 7 and 8). (C) Co-immunoprecipitation of Nuf2p and Spc24p with Spc25p-6HA. The experimental design was as in (B), using SPC25 (lanes 1, 3 and 5) and SPC25-3HA cell extract (lanes 2, 4 and 6). The precipitates were tested with anti-HA (lanes 1 and 2), anti-Spc24p (lanes 3 and 4) and anti-C-Nuf2p antibodies (lanes 5 and 6).
To confirm complex formation of these proteins, Ndc80p-6HA and Spc25p-3HA were immunoprecipitated using anti-haemagglutinin (HA) antibodies. As shown by immunoblotting, Spc24p (Figure 1B, lane 8, and C, lane 4) co-immunoprecipitated with Ndc80p-6HA (Figure 1B, lane 2) and Spc25p-3HA (Figure 1C, lane 2). We also excluded the possibility that entire SPBs or larger SPB subcomplexes were precipitated by the anti-HA antibodies. Spc72p, a component of the outer plaque (Knop and Schiebel, 1998), Spc110p, which binds to the γ-tubulin complex at the inner plaque (Knop and Schiebel, 1997), γ-tubulin (Tub4p) (Spang et al., 1996) and β-tubulin (Tub2p) (Neff et al., 1983) were not detected in the immunoprecipitate (not shown). Surprisingly, Nuf2p, an SPB-associated protein (Osborne et al., 1994) was identified in the Ndc80p-6HA precipitate by antibodies directed against the N-terminal (Figure 1B, lane 6) and C-terminal domain of Nuf2p (Figure 1B, lane 4). This suggested that Nuf2p interacts with Ndc80p, Spc24p or Spc25p. Nuf2p was probably not found in the Spc25p–ProA purification (Figure 1A, lane 2) because the eluted IgG heavy chain overlapped with the 53 kDa Nuf2p band. To confirm this notion, the Spc25p-3HA precipitate was tested for Nuf2p. Indeed, Nuf2p (Figure 1C, lane 6) co-immunoprecipitated with Spc25p-3HA (lane 2). All these precipitations were specific, since no co-immunoprecipitations were observed when control strains without a tag were used (Figure 1B, lanes 1, 3, 5 and 7, and C, lanes 1, 3 and 5). In conclusion, Ndc80p, Nuf2p, Spc25p and Spc24p are present in common complexes.
NDC80, NUF2, SPC24 and SPC25 function together
To confirm that NDC80, NUF2, SPC25 and SPC24 function together in vivo, we investigated whether the four genes interact genetically. Multiple genetic interactions were observed when conditional lethal NDC80, NUF2, SPC24 or SPC25 cells were tested for high gene dosage suppression (Table I). The growth defect of spc24-3 and spc25-4 cells at 37°C was strongly suppressed by high gene dosage of SPC25 or SPC24, respectively. In contrast, multiple copies of SPC25 suppressed the growth defect of spc24-2 cells only at 33°C, but not at higher temperatures (Table I), indicating that the extent of suppression is allele specific. Somewhat weaker suppressions were observed of nuf2-61 cells by SPC24 and SPC25, of ndc80-1 cells by SPC24 and SPC25, of spc25-4 cells by NDC80, and of spc25-7 cells by NDC80 and SPC24. As a control for the specificity of the suppression, we tested SPC98, coding for a subunit of the yeast γ-tubulin complex that is located at the inner plaque of the SPB, as are Ndc80p, Spc24p and Spc25p (Geissler et al., 1996). SPC98 had no influence on the growth of the temperature-sensitive ndc80(ts), nuf2(ts), spc24(ts) or spc25(ts) cells (Table I).
Table I. Genetic interactions of NDC80, NUF2, SPC24 and SPC25.
| Mutant | pRS426 with | Growth at the indicated temperature |
||||
|---|---|---|---|---|---|---|
| 23°C | 30°C | 33°C | 35°C | 37°C | ||
| nuf2-61 | NDC80 | +++ | +++ | – | – | – |
| NUF2 | +++ | +++ | +++ | +++ | +++ | |
| SPC24 | +++ | +++ | + | – | – | |
| SPC25 | +++ | +++ | + | – | – | |
| SPC98 | +++ | +++ | – | – | – | |
| no insert | +++ | +++ | – | – | – | |
| ndc80-1 | NDC80 | +++ | +++ | +++ | +++ | +++ |
| NUF2 | +++ | +++ | – | – | – | |
| SPC24 | +++ | +++ | + | – | – | |
| SPC25 | +++ | +++ | + | – | – | |
| SPC98 | +++ | +++ | – | – | – | |
| no insert | +++ | +++ | – | – | – | |
| ndc80-2 | NDC80 | +++ | +++ | +++ | +++ | +++ |
| NUF2 | +++ | +++ | +++ | – | – | |
| SPC24 | +++ | +++ | +++ | + | – | |
| SPC25 | +++ | +++ | +++ | – | – | |
| SPC98 | +++ | +++ | +++ | – | – | |
| no insert | +++ | +++ | +++ | – | – | |
| spc24-2 | NDC80 | +++ | +++ | + | – | – |
| NUF2 | +++ | +++ | + | – | – | |
| SPC24 | +++ | +++ | +++ | +++ | +++ | |
| SPC25 | +++ | +++ | +++ | – | – | |
| SPC98 | +++ | +++ | + | – | – | |
| no insert | +++ | +++ | + | – | – | |
| spc24-3 | NDC80 | +++ | +++ | +++ | + | – |
| NUF2 | +++ | +++ | +++ | + | – | |
| SPC24 | +++ | +++ | +++ | +++ | +++ | |
| SPC25 | +++ | +++ | +++ | +++ | +++ | |
| SPC98 | +++ | +++ | +++ | + | – | |
| no insert | +++ | +++ | +++ | + | – | |
| spc25-4 | NDC80 | +++ | +++ | +++ | + | – |
| NUF2 | +++ | +++ | +++ | – | – | |
| SPC24 | +++ | +++ | +++ | +++ | +++ | |
| SPC25 | +++ | +++ | +++ | +++ | +++ | |
| SPC98 | +++ | +++ | +++ | – | – | |
| no insert | +++ | +++ | +++ | – | – | |
| spc25-7 | NDC80 | +++ | +++ | + | – | – |
| NUF2 | +++ | +++ | – | – | – | |
| SPC24 | +++ | +++ | + | – | – | |
| SPC25 | +++ | +++ | +++ | +++ | +++ | |
| SPC98 | +++ | +++ | – | – | – | |
| no insert | +++ | +++ | – | – | – | |
High gene dosage suppression was tested as described in Materials and methods.
–, no growth; +, reduced growth; +++, normal growth.
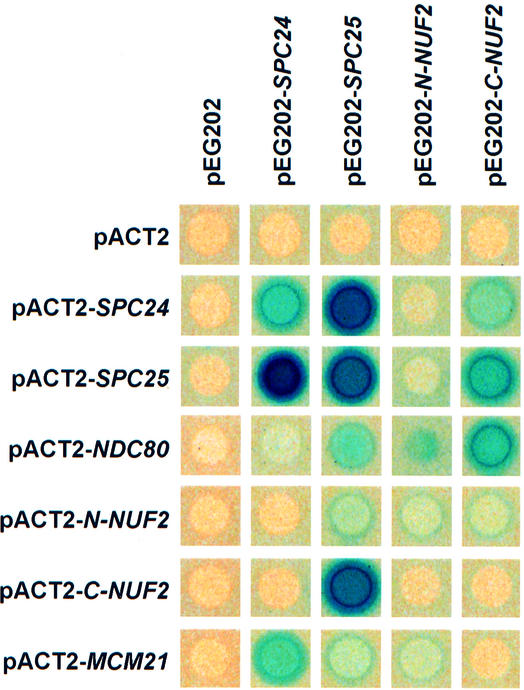
Strong two-hybrid interactions were detected between Ndc80p and the C-terminal domain of Nuf2p (C-Nuf2p), Spc24p and Spc25p, and C-Nuf2p and Spc25p. C-Nuf2p and Spc24p, Ndc80p and Spc25p, and Ndc80p and N-Nuf2p showed weaker interactions (Figure 2). Some of these two-hybrid interactions have been identified previously (Cho et al., 1998). When taken together, our genetic data suggest that Ndc80p, Nuf2p, Spc24p and Spc25p function together in vivo.
Fig. 2. Ndc80p, Nuf2p, Mcm21p, Spc24p and Spc25p interact in the yeast two-hybrid system. Yeast strains containing the indicated yeast two-hybrid plasmids were overlayed with top agar containing 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) to measure β-galactosidase activity. Plates were incubated for 6 h at 30°C. Blue colour indicates interaction.
spc24-2 and spc25-7 cells are defective in chromosome segregation and checkpoint control
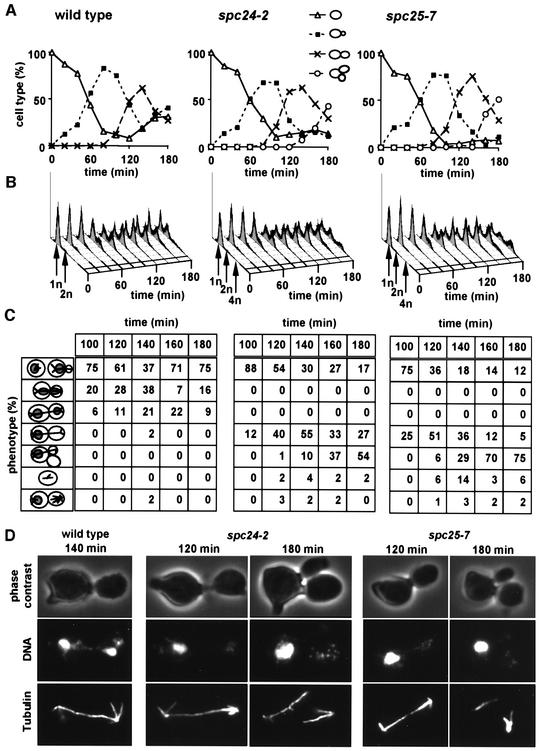
The function of SPC24 and SPC25 was investigated by analysing the phenotype of conditional lethal mutants. Synchronized wild-type, spc24-2 and spc25-7 cells (t = 0) were shifted to 37°C. spc24-2 and spc25-7 cells replicated their DNA and formed a bud with similar kinetics to wild-type cells (Figure 3A–C). However, in contrast to wild-type cells, chromosome segregation of spc24-2 and spc25-7 cells was disturbed (Figure 3C and D). In wild-type cells, separation of DNA masses coincided with the formation of an anaphase spindle (Figure 3D, wild type). In spc24-2 (Figure 3D, spc24-2, 120 min) and spc25-7 cells (Figure 3D, spc25-7, 120 min), the chromosomal DNA remained in the mother cell (cell with mating projection), despite the formation of an anaphase spindle. Moreover, many spc24-2 and spc25-7 cells did not arrest in the cell cycle, as indicated by the re-replication of the DNA (Figure 3B, 4n peak after 160–180 min) and the formation of an additional bud (Figure 3C and D, spc24-2 and spc25-7, 180 min). Thus, spc24-2 and spc25-7 cells are defective in chromosome segregation and fail to arrest in the cell cycle.
Fig. 3. spc24-2 and spc25-7 cells form an anaphase spindle but fail to segregate the chromosomes. Wild-type, spc24-2 and spc25-7 cells were synchronized with α-factor. The block was released and the cells were shifted to the restrictive temperature. Samples were withdrawn every 20 min and analysed for (A) budding index and (B) DNA content by flow cytometry. (C and D) Indirect immunofluorescence with anti-tubulin antibodies and 4′-6-diamidine-2-phenylindole (DAPI) staining. (C) is the quantification of (D) (n = 200). (C) In the cartoons at the left side of the panel, the grey circles within the cells symbolize the DAPI staining regions, and the lines the microtubules. Note that only the cell types after 100 min incubation at 37°C are shown.
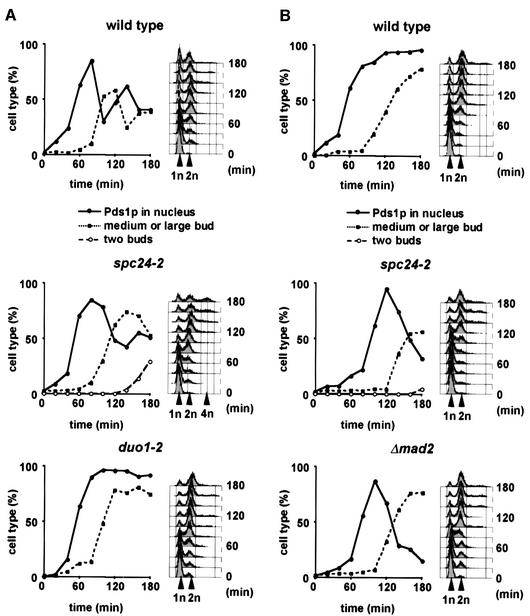
The degradation of Pds1p via the anaphase promoting complex and the proteasome at the metaphase to anaphase transition is inhibited upon activation of the Mad2p-dependent checkpoint (hereafter named the Mad2p checkpoint) (Hwang et al., 1998). To determine whether spc24-2 cells are Mad2p checkpoint deficient, we investigated the kinetics of Pds1p degradation of synchronized PDS1-6HA and spc24-2 PDS1-6HA cells incubated at 37°C. duo1-2 PDS1-6HA cells, which arrest in metaphase due to Mad2p checkpoint activation (Hofmann et al., 1998), were used as a control. Whereas duo1-2 cells arrested in the cell cycle with stable Pds1p after ∼80 min, wild-type cells degraded the nuclear Pds1p before the occurrence of large buds (Figure 4A, wild type, decline of Pds1p after 80 min). spc24-2 cells also degraded the nuclear Pds1p, but were slightly delayed by ∼20 min compared with wild-type cells (Figure 4A, spc24-2, Pds1p strongly declined after 100 min). Furthermore, re-replication and bud formation of spc24-2 cells further indicated cell cycle progression (see flow cytometry and cells with two buds in Figure 4A, spc24-2). Together, these results suggest that the mitotic checkpoint is impaired in spc24-2 cells.
Fig. 4. spc24-2 cells are checkpoint deficient. (A and B) α-factor synchronized wild-type, spc24-2, duo1-2 or Δmad2 cells containing PDS1-6HA were shifted to 37°C with (B) or without (A) 15 µg/ml nocodazole. Samples were withdrawn every 20 min and analysed for budding index, nuclear Pds1p by immunofluorescence, and DNA content by flow cytometry. Cells were also stained with anti-tubulin antibodies to ensure microtubule depolymerization when incubated with nocodazole.
The checkpoint defect of spc24-2 cells was investigated further by following cell cycle progression of synchronized PDS1-6HA, spc24-2 PDS1-6HA and Δmad2 PDS1-6HA cells at 37°C in the presence of the microtubule depolymerizing drug nocodazole. As reported before (Yamamoto et al., 1996), most nocodazole-treated wild-type cells arrested in the cell cycle with a large bud, replicated DNA and stable Pds1p in response to the Mad2p checkpoint (Figure 4B). In contrast, Δmad2 cells failed to arrest at the metaphase to anaphase transition, as indicated by the degradation of nuclear Pds1p. Δmad2 cells finally arrested at the end of anaphase because of an alternative checkpoint (Bub2p dependent), which is regulated by the movement of the SPB into the bud (Bardin et al., 2000; Pereira et al., 2000). SPB migration into the bud does not take place when microtubules are depolymerized. Importantly, spc24-2 cells behaved very similarly to Δmad2 cells, confirming that the Mad2p checkpoint function is defective. Collectively, these results demonstrate that Spc24p, and possibly also Ndc80p and Spc25p, are important for the Mad2p checkpoint function.
Ndc80p, Nuf2p, Spc24p and Spc25p bind to CEN3 DNA, and SPC24 interacts with MCM21
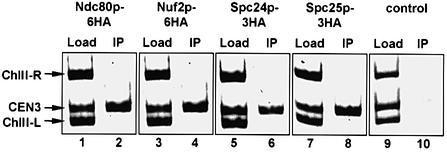
The chromosome segregation defect of ndc80-1 (Wigge et al., 1998), spc24-2 and spc25-7 cells is similar to that of ndc10-1 cells (Goh and Kilmartin, 1993). Since Ndc10p is a component of the CBF3 kinetochore complex (Lechner and Carbon, 1991), we considered the possibility that the Ndc80p, Nuf2p, Spc24p and Spc25p proteins interact with the kinetochore. This notion was tested by chromatin immunoprecipitation (ChIP) using fully functional, chromosomally tagged NDC80-6HA, NUF2-6HA, SPC24-3HA and SPC25-3HA gene fusions. CEN3 DNA was co-immunoprecipitated with Ndc80p-6HA (Figure 5, lane 2), Nuf2p-6HA (lane 4), Spc24p-3HA (lane 6) and Spc25p-3HA (lane 8) by the anti-HA antibodies. These precipitations were found to be specific for CEN3 because CEN3 and control DNAs flanking CEN3 (ChIII-R and ChIII-L) (Ortiz et al., 1999) were detected equally in the input (Figure 5, Load, lanes 1, 3, 5 and 7), but only CEN3 was strongly enriched in the immunoprecipitates (Figure 5, IP, lanes 2, 4, 6 and 8). To exclude unspecific CEN3–antibody interactions, a wild-type strain devoid of an HA epitope was processed equally (Figure 5, lanes 9 and 10). In conclusion, the ChIP results suggest that Ndc80p, Nuf2p, Spc24p and Spc25p are components of the kinetochore.
Fig. 5. Ndc80p, Nuf2p, Spc24p and Spc25p are associated with the budding yeast kinetochore. NDC80-6HA (lanes 1 and 2), NUF2-6HA (lanes 3 and 4), SPC24-3HA (lanes 5 and 6) and SPC25-3HA cells (lanes 7 and 8), and wild-type cells without an HA tag (lanes 9 and 10) were subjected to ChIP. The immunoprecipitation was tested for the presence of CEN3 DNA and fragments left (ChIII-L) and right (ChIII-R) of CEN3 (IP: lanes 2, 4, 6, 8 and 10), as described previously (Ortiz et al., 1999). As a positive control for the PCR reactions, yeast lysates were tested without anti-HA precipitation (Load: lanes 1, 3, 5, 7 and 9).
This finding is supported by the genetic (Table II) and two-hybrid interactions (Figure 2) of SPC24 with MCM21 coding for a subunit of the kinetochore (Ortiz et al., 1999). However, attempts to co-immunoprecipitate Mcm21p and Spc24p failed (not shown). This may reflect the fact that both proteins are part of different kinetochore subcomplexes, which may be dissolved upon extraction of the proteins during the immunoprecipitation. Alternatively, the interaction between Spc24p and Mcm21p may be indirect. In summary, SPC24 interacts genetically with the kinetochore component MCM21.
Table II. MCM21 and SPC24 interact genetically.
| Genotype | Growth at the indicated temperature |
||||
|---|---|---|---|---|---|
| 23°C | 30°C | 33°C | 35°C | 37°C | |
| Δmcm21 | +++ | +++ | +++ | +++ | +++ |
| ndc80-1 | +++ | +++ | + | – | – |
| ndc80-1 Δmcm21 | +++ | +++ | + | – | – |
| nuf2-61 | +++ | +++ | – | – | – |
| nuf2-61 Δmcm21 | +++ | +++ | – | – | – |
| spc24-2 | +++ | +++ | + | – | – |
| spc24-2 Δmcm21 | – | – | – | – | – |
| spc24-3 | +++ | +++ | +++ | +++ | – |
| spc24-3 Δmcm21 | +++ | +++ | +++ | – | – |
| spc25-4 | +++ | +++ | +++ | + | – |
| spc25-4 Δmcm21 | +++ | +++ | +++ | + | – |
| spc25-7 | +++ | +++ | + | – | – |
| spc25-7 Δmcm21 | +++ | +++ | – | – | – |
Synthetic lethality was determined as described in Materials and methods. –, no growth; +, reduced growth; +++, normal growth on 5-FOA plates. No growth of spc24-2 Δmcm21 cells at 23 and 30°C indicates synthetic lethality since spc24-2 or Δmcm21 cells grew at these temperatures.
Nuf2p co-localizes with the centromere
To confirm the result of the ChIP analysis, we investigated by immunofluorescence microscopy the localization of Nuf2p, the kinetochore protein Mcm21p (Ortiz et al., 1999) and the SPB component Spc98p (Geissler et al., 1996) relative to CEN5 DNA. Owing to the clustering of the centromeres over most parts of the cell cycle (Jin et al., 2000), kinetochore proteins are observed by immunofluorescence as one or two dots per cell, located close to the nuclear side of the SPB represented by the γ-tubulin complex (Goshima and Yanagida, 2000). Thus, if Nuf2p is a kinetochore component, it should co-localize with CEN5 DNA but behave differently to the γ-tubulin complex protein Spc98p.
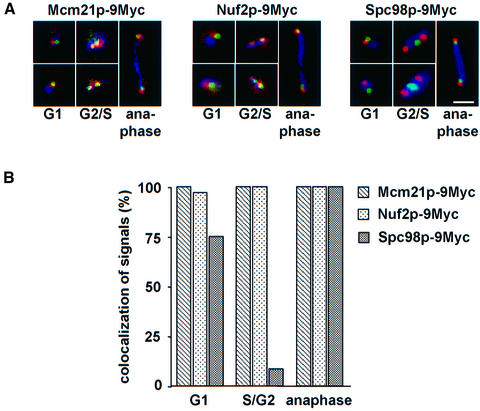
For this study, a strain was constructed in which arrays of TetO binding sites were introduced next to CEN5 DNA (He et al., 2000). In this strain, MCM21, NUF2 and SPC98 were tagged with nine copies of the Myc-epitope. The localization of the three proteins relative to CEN5 was determined in fixed cells. The established kinetochore protein Mcm21p (Figure 6A, Mcm21p-9Myc, red) and Nuf2p (Figure 6A, Nuf2p-9Myc, red) were detected as a dot, which in the vast majority of cells (Figure 6B, >95%) was located <0.4 µm from the green CEN5. A complete overlap between the CEN5 and Mcm21p or Nuf2p signals was only observed in ∼30% of the cells. However, a perfect co-localization was not always expected since the CEN5 signal reflects a single centromere, whereas the Nuf2p or Mcm21p signals represent the average of 16 centromeres. Importantly, Nuf2p-9Myc and Mcm21p-9Myc localization relative to CEN5 DNA was independent of whether the cells were in G1, S/G2 or anaphase (Figure 6B). In contrast, in 90% of S/G2 cells the red signal of the SPB component Spc98p-9Myc was clearly distinct (≥0.4 µm apart) from the green CEN5 (Figure 6A, Spc98p-9Myc, G2/S, and B). However, Spc98p-9Myc was found in most G1 phase (Figure 6A, Spc98p-9Myc, G1, upper panel) and anaphase cells within 0.4 µm of the CEN5 DNA dot (Figure 6A, Spc98p-9Myc, anaphase), indicating that during these stages of the cell cycle kinetochores are clustered very close to the SPB. In ∼25% of G1 phase cells, the Spc98p-9Myc and CEN5 signals were clearly different (Figure 6A, Spc98p-9Myc, G1, lower panel). In conclusion, the localization of Nuf2p relative to CEN5 DNA was similar to that of the kinetochore protein Mcm21p, but distinct from the SPB component Spc98p, providing further evidence that Nuf2p is located at the yeast kinetochore.
Fig. 6. Nuf2p and Mcm21p are located close to the CEN5. (A) Localization of Mcm21p-9Myc, Nuf2p-9Myc and Spc98p-9Myc relative to CEN5 DNA. Synchronized cells with GFP-labeled CEN5 DNA carrying MCM21-9Myc, NUF2-9Myc or SPC98-9Myc were fixed and analysed by direct and indirect immunofluorescence for CEN5 DNA (green), Myc-tagged protein (red) and tubulin (blue). Note that the overlap between the green and red signals appears in yellow. Shown are cells in G1 phase (monopolar spindle), G2/S phase (short spindle) and anaphase (long spindle) of the cell cycle. Bar: 5 µm. (B) Quantification of (A). Cells (n = 100) in G1, G2/S phase or anaphase of the cell cycle were analysed. Signals were counted as ‘co-localized’ when the CEN5 signal (green) was <0.4 µm from the red anti-HA dot.
Kinetochore association of Ndc80p and Spc24p is defective in ndc10-1 cells
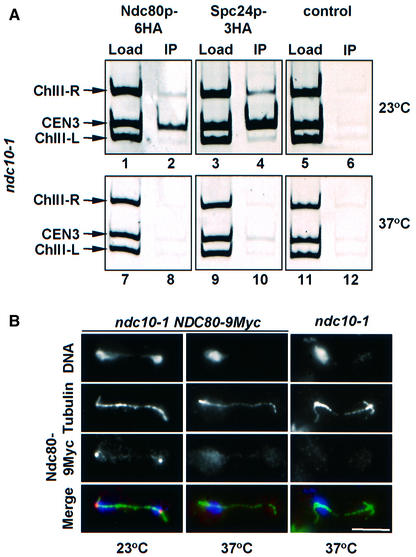
In ndc10-1 cells, the centromere DNA is devoid of most if not all kinetochore proteins (Goh and Kilmartin, 1993; Ortiz et al., 1999; Goshima and Yanagida, 2000). Therefore, we would expect that in ndc10-1 cells Ndc80p and Spc24p are no longer associated with the centromere at the restrictive temperature. ndc10-1 NDC80-6HA (Figure 7A, lanes 1, 2, 7 and 8), ndc10-1 SPC24-3HA (lanes 3, 4, 9 and 10) and ndc10-1 cells (control, lanes 5, 6, 11 and 12) were analysed by ChIP. The CEN3 DNA was enriched with Ndc80p-6HA or Spc24p-3HA of ndc10-1 NDC80-6HA or ndc10-1 SPC24-3HA cells grown at 23°C (lanes 2 and 4, compare Load with IP) but not at 37°C (lanes 8 and 10). No precipitation of CEN3 DNA was observed with the ndc10-1 control cells at any temperature (lanes 6 and 12).
Fig. 7. Localization of Ndc80p and Spc24p in ndc10-1 cells. (A) Ndc80p and Spc24p are not associated with the kinetochore in ndc10-1 cells incubated at the restrictive temperature. Cells of ndc10-1 NDC80-6HA (lanes 1, 2, 7 and 8), ndc10-1 spc24-3HA (lanes 3, 4, 9 and 10) and ndc10-1 (control; lanes 5, 6, 11 and 12) grown at 23°C (lanes 1–6) were shifted to 37°C for 4 h (lanes 7–12). CEN3 association of Ndc80p-6HA (lanes 2 and 8) and Spc24p-3HA (lanes 4 and 10) was investigated by ChIP. ndc10-1 cells were employed as a control for the specificity of the immunoprecipitation (lanes 6 and 12) as well as binding to regions left (ChIII-L) and right (ChIII-R) of CEN3 DNA. Yeast lysates were also tested without precipitation (Load: lanes 1, 3, 5, 7, 9 and 11). (B) Cells of ndc10-1 NDC80-9Myc and ndc10-1 NDC80 were shifted from 23 to 37°C for 4 h. Fixed cells were analysed by indirect immunofluorescence using anti-tubulin and anti-Myc antibodies. DNA was stained with DAPI. The bottom panel shows the merged signals of the DNA (blue), tubulin (green) and Ndc80p-9Myc (red) stainings. Bar: 5 µm.
This result was confirmed by the analysis of ndc10-1 NDC80-9Myc and ndc10-1 SPC24-GFP cells (where GFP is green fluorescent protein) using immunofluorescence microscopy. At 23°C, Ndc80p-9Myc (Figure 7B, ndc10-1 NDC80-9Myc) and Spc24p-GFP (not shown) were detected as single dots situated close to the spindle pole. In contrast, in ndc10-1 cells incubated at 37°C, both Ndc80p-9Myc (Figure 7B, ndc10-1 NDC80-9Myc) and Spc24p-GFP (not shown) localized diffusely in the nucleus. No signal was detected with the anti-Myc antibody when ndc10-1 cells were used (Figure 7B, panel ndc10-1), indicating that the Ndc80p-9Myc signal in ndc10-1 NDC80-9Myc cells was specific. Therefore, both ChIP and immunofluorescence microscopy indicate that the localization of Ndc80p and Spc24p with the kinetochore is dependent on Ndc10p.
Kinetochores are no longer clustered in spc24-2 and spc25-7 cells
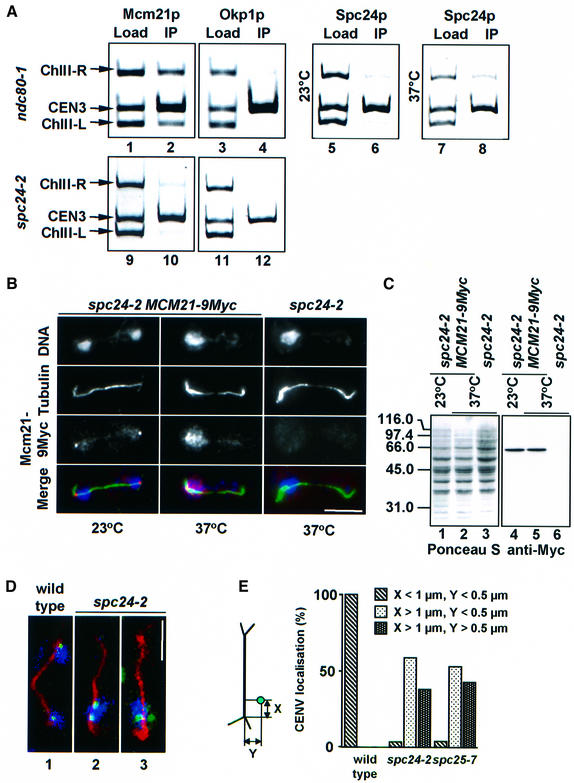
We investigated by ChIP whether mutations in NDC80 or SPC24 affect the overall structure of the yeast kinetochore. When ndc80-1 (Figure 8A, lanes 1–8) or spc24-2 cells (lanes 9–12) were shifted to 37°C, the core kinetochore proteins Ctf13p, Ctf19p (data not shown), Mcm21p (lanes 2 and 10) and Okp1p (lanes 4 and 12) as well as Spc24p (lane 8) were still associated with CEN3 DNA. The relative Spc24p signals (ratio of load/IP) at 23°C (lanes 5 and 6) and 37°C (lanes 7 and 8) were about the same (0.9 versus 0.8), suggesting that Spc24p localization with CEN3 was hardly affected in ndc80-1 cells. When taken together, these results suggest that the kinetochore is still partially intact in ndc80-1 and spc24-2 cells.
Fig. 8. Kinetochores are no longer clustered near SPBs in spc24-2 cells. (A) Mcm21p, Okp1p and Spc24p are associated with CEN3 DNA in ndc80-1 and spc24-2 cells. Cells of ndc80-1 (lanes 1–8) and spc24-2 (lanes 9–12) were tested for Mcm21p (lanes 1, 2, 9 and 10), Okp1p (lanes 3, 4, 11 and 12) and Spc24p (lanes 5–8) CEN3 DNA localization by ChIP at 23°C (lanes 5 and 6), or after 3 h at 37°C (lanes 1–4 and 7–12) using anti-Mcm21p (lanes 2 and 10), anti-Okp1p (lanes 4 and 12) or anti-Spc24p antibodies (lanes 6 and 8). Binding to regions left (ChIII-L) and right (ChIII-R) of CEN3 was tested as controls. Yeast lysates were assayed without precipitation (Load: lanes 1, 3, 5, 7, 9 and 11). (B) Localization of Mcm21p-9Myc in spc24-2 cells. Cells of spc24-2 MCM21-9Myc and spc24-2 were shifted from 23 to 37°C for 3 h. Fixed cells were analysed by indirect immunofluorescence using anti-Myc and anti-tubulin antibodies. DNA was stained with DAPI. The three signals were merged to compare localization. DAPI is shown in blue, the Mcm21p signal in red and microtubules in green. (C) Mcm21p levels remain constant. spc24-2 (lanes 3 and 6) and spc24-2 MCM21-9Myc cells (lanes 1, 2, 4 and 5) were grown at 23°C. One-half of the culture was incubated for 3 h at 37°C (lanes 2, 3, 5 and 6). Cell extracts were separated by SDS–PAGE and transferred to a nitrocellulose membrane. The membrane was stained with Ponceau S (lanes 1–3) to visualize proteins and then processed with anti-Myc antibodies (lanes 4–6). (D and E) Synchronized wild-type, spc24-2 and spc25-7 cells with GFP-labelled CEN5 DNA were shifted to 37°C. Cells were processed by anti-tubulin immunofluorescence and then analysed by confocal microscopy. (D) Large budded wild-type (panel 1) or spc24-2 cells (panels 2 and 3) containing an anaphase spindle. Tubulin is shown in red, CEN5 in green and DAPI in blue. (E) The position of CEN5 (green dot in cartoon) relative to the spindle pole (intersection of astral and nuclear microtubules) in the mother cell body of cells with an anaphase spindle was determined. Each CEN5 signal was counted separately. At least 100 cells were analysed. (B and D) Bars: 5 µm.
spc24-2 and spc25-7 cells may fail to segregate the chromosomes because the kinetochore is defective in bipolar attachment to microtubules, binding to microtubules or clustering at the SPB. To elucidate the kinetochore defect of spc24-2 and spc25-7 cells, we investigated the localization of the kinetochore protein Mcm21p. The Mcm21p-9Myc signal was situated ‘dot-like’ close to spindle poles, indicative of centromere clustering, in spc24-2 cells at 23°C (Figure 8B, spc24-2 MCM21-9Myc). When spc24-2 cells were shifted to 37°C, however, the Mcm21p-9Myc staining was no longer clustered. Instead, the Mcm21p-9Myc signal was dispersed within the DAPI staining region in the mother cell body (Figure 8B, spc24-2 MCM21-9Myc). The Mcm21p-9Myc signal was found to be specific since only a faint background signal was observed when spc24-2 cells were incubated with the anti-Myc antibodies (Figure 8B, spc24-2). Furthermore, we established by immunoblotting that the dispersed Mcm21p-9Myc signal of spc24-2 cells at 37°C was not the result of increased Mcm21p levels (Figure 8C, compare lanes 4 and 5).
The failure of kinetochores to cluster close to the SPB was confirmed by using spc24-2 and spc25-7 cells in which TetO binding sites were introduced adjacent to CEN5. In anaphase wild-type cells at 37°C, CEN5 was always found close to the spindle poles (Figure 8D, panel 1, and 8E). In contrast, in 98% of the spc24-2 and spc25-7 cells, CEN5 was located in the nucleoplasm of the mother cell body either close to (Figure 8D, panel 2) or >0.5 µm away from (panel 3) microtubules, but not near the spindle poles. Furthermore, >50%, approximately, of the CEN5 signals of spc24-2 and spc25-7 cells appeared as two discrete dots. This result is consistent with our previous finding (Figure 4) indicating that spc24-2 cells degrade Pds1p and therefore probably also the cohesin Scc1p, the latter being responsible for sister chromatid cohesion in metaphase (Uhlmann et al., 2000). In conclusion, although the core kinetochore structure is at least partially intact in spc24-2 cells, the kinetochores fail to cluster close to the spindle poles. Since microtubuli have been described to be important for centromere clustering (Jin et al., 2000), one explanation for the clustering defect is a lack of microtubule attachment.
Discussion
Ndc80p, Nuf2p, Spc24p and Spc25p belong to a group of proteins that have been co-purified with the budding yeast spindle and localize at or adjacent to the SPB (Rout and Kilmartin, 1990; Wigge et al., 1998). In this study we show that the four proteins interact, a conclusion that is based on the co-purification of Ndc80p and Spc24p with Spc25p–ProA, the co-immunoprecipitation of the four proteins, and two-hybrid and genetic interactions of NDC80, NUF2, SPC24 and SPC25. An interaction of Ndc80p and Nuf2p is also consistent with the co-localization of both proteins observed by indirect immunofluorescence (Osborne et al., 1994).
Ndc80p, Spc24p and Spc25p have been localized by immunoelectron microscopy close to the nuclear inner plaque of the SPB (Wigge et al., 1998). After the observation that centromeres are clustered in the vicinity of the SPB (Goh and Kilmartin, 1993; Hyland et al., 1999; Goshima and Yanagida, 2000; He et al., 2000; Jin et al., 2000; Tanaka et al., 2000), it became feasible that the cellular localization of Ndc80p, Spc24p and Spc25p reflects clustered centromeres. Here we provide evidence that Ndc80p, Spc24p, Spc25p and Nuf2p are indeed associated with the kinetochore. First, ChIP experiments clearly demonstrate that the four proteins are localized to the centromere DNA in an Ndc10p-dependent manner. Secondly, immunofluorescence microscopy detects Nuf2p close to CEN5 DNA. Thirdly, MCM21 interacts genetically with SPC24. Finally, the phenotype of conditional lethal mutants (discussed below) is consistent with a kinetochore function of Ndc80p, Spc24p and Spc25p. Our conclusion is supported further by the conditional synthetic lethal effect of ndc80-1 and Δctf19 [CTF19 codes for a kinetochore protein (Hyland et al., 1999)] and by the finding that the human homologue of Ndc80p, HEC1, localizes to the centromere (Chen et al., 1997).
Kinetochores provide the attachment sites for spindle microtubules, are required for centromere DNA clustering at the SPB, regulate progress through mitosis upon spindle attachment, possibly sense bipolarity and are a prerequisite for cohesin complex formation in the centromere region of the chromosome (Pidoux and Allshire, 2000). A complete disruption of the centromere, as in ndc10-1 cells, is likely to eliminate all of these functions, but this is not the case in ndc80-1 and spc24-2 cells because these mutants maintain partially assembled kinetochores, as judged from ChIP experiments. Therefore, we propose that the phenotypes of ndc80-1, spc24-2 and spc25-7 cells indicate specific functions of the encoded proteins at the kinetochore.
The phenotypes of ndc80-1 (Wigge et al., 1998), spc24-2 and spc25-2 cells (this study) were remarkably similar. All the mutant cells formed an anaphase spindle of wild-type morphology, which failed to segregate the duplicated chromosomes. The reason for the chromosome segregation defect is not fully understood. Our data reveal that kinetochores are no longer clustered at SPBs in spc24-2 and spc25-7 cells. This indicates that either the kinetochores fail to bind to microtubules or that a direct interaction between the kinetochore and the SPB is defective. Notably, kinetochores of spc24-2 and spc25-7 cells were often (in ∼55% of cases) found close to the anaphase spindle, suggesting that some kinetochores may have microtubule binding activity. In any case, microtubule binding of spc24-2 and spc25-7 kinetochores must be impaired, since ∼45% of the centromeres were located >0.5 µm from microtubules.
It is possible that the binding of Ndc80p, Spc24p and Spc25p to SPB components assists chromosome segregation during anaphase B. In favour of this model is the clustering of centromeres along the nuclear face of the SPB (Goshima and Yanagida, 2000; this study), the co-purification of Ndc80p, Spc24p and Spc25p with the SPB, whereas other core kinetochore proteins like Ndc10p were not identified in this preparation (Wigge et al., 1998), and the finding that microtubule depolymerization affects centromere clustering only moderately (Jin et al., 2000). Notably, Spc24p and Spc25p interact with core SPB components, as seen in the yeast two-hybrid system (Newman et al., 2000; C.Janke, unpublished), although as yet we have been unable to confirm these interactions by co-immunoprecipitation. However, an argument against a prolonged interaction of kinetochore components and the SPB is the rapid change in distance between the centromere and the SPB, as seen by time-lapse experiments using the central SPB component Spc42p and CEN5 DNA as markers (He et al., 2000; Tanaka et al., 2000). Some of these changes may be attributed to the stretching of Spc110p, a filamentous SPB component that connects the γ-tubulin complex to the central Spc42p layer (Kilmartin et al., 1993; Knop and Schiebel, 1997).
A defect in spindle–kinetochore interaction should trigger the Mad2p checkpoint, which arrests cells in metaphase with unseparated sister chromatids and a short spindle (Hwang et al., 1998). Consistently, cells of ctf13-30, Δctf19 and mtw1-1 (Spencer and Hieter, 1992; Hyland et al., 1999; Goshima and Yanagida, 2000) stop cell cycle progression at the metaphase to anaphase transition in a Mad2p-dependent manner (Wang and Burke, 1995). In contrast, spc24-2 cells were only slightly delayed in anaphase entry despite their complete failure to segregate the chromosomes, suggesting a checkpoint defect. This notion is further supported by the observation that spc24-2 cells at 37°C did not arrest at the metaphase to anaphase transition when microtubules were depolymerized.
The kinetochore of mammalian cells provides an essential scaffold for the binding and function of the checkpoint proteins BUB1 (Taylor and McKeon, 1997), BUB3 (Taylor et al., 1998), MAD2 (Chen et al., 1996) and BUBR1 (Chan et al., 1999). Centromere proteins such as CENP-E have been found to interact with BUBR1 and are essential for checkpoint control (Abrieu et al., 2000). Similarly, Ndc80p, Spc24p and Spc25p may provide an essential scaffold that allows Mad2p checkpoint components to assemble at the kinetochore and which may be crucial for the sensing of microtubule attachment defects. In this respect, it is interesting that a two-hybrid interaction has been reported for Mad1p and Spc25p (Newman et al., 2000).
The phenotype of nuf2-61 cells was less informative. They arrested in the cell cycle with a short metaphase-like spindle (Osborne et al., 1994) in response to the Mad2p checkpoint (E.Schiebel, unpublished). This arrest phenotype indicates that nuf2-61 cells are still checkpoint proficient. Analysis of additional nuf2(ts) alleles will reveal whether NUF2 has the same or merely an overlapping function with NDC80, SPC24 and SPC25.
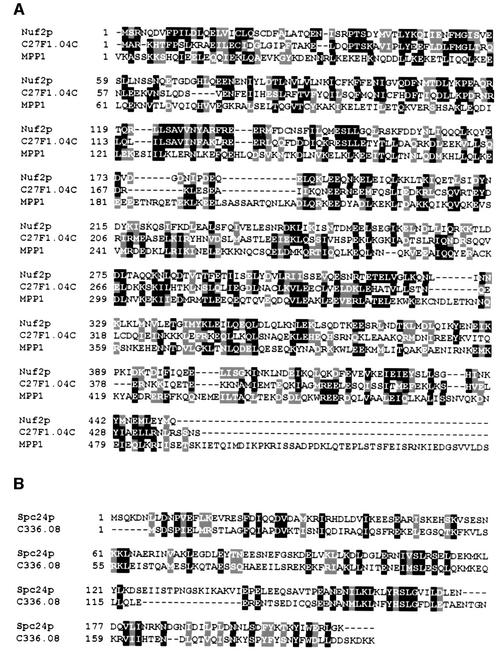
Homologues of Ndc80p and Nuf2p have been identified from fission yeast to mammalian cells. Ndc80p shares 30% identity with mammalian HEC1 (Wigge et al., 1998). A similar function of the HEC1 protein and Ndc80p is suggested by the finding that both proteins associate with the kinetochore and are important for chromosome segregation (Chen et al., 1997; Wigge et al., 1998). Importantly, deletion of the essential yeast NDC80 is fully complemented by the human HEC1, demonstrating the close functional relationship of both genes (Zheng et al., 1999). Databank searches revealed that the human MPP1 protein is 25% identical to Nuf2p over 280 amino acids (Figure 9A). MPP1 has been identified as an M-phase nuclear phosphoprotein recognized by the MPM2 monoclonal antibody (Westendorf et al., 1994; Fritzler et al., 2000). In addition, Schizosaccharomyces pombe proteins of unknown function with 25% identity to Nuf2p (Figure 9A) or Spc24p (Figure 9B) are present in the database. When taken together, it is likely that homologues of Ndc80p, Nuf2p and Spc24p interact in other organisms. These proteins may also be associated with kinetochores and function in the attachment of kinetochores to microtubules and in checkpoint control.
Fig. 9. Alignment of homologues of Nuf2p and Spc24p (program Clustal_X; Thompson et al., 1997). Homologous proteins were identified in a BLAST search (program WU-blastp; database, swall; matrix, blosum62; Altschul and Gish, 1996) using the protein sequence of Nuf2p and Spc24p. (A) The S.pombe homologue of Nuf2p, C27F1.04C [P(N) of 1.1 × 10–27; high score of 318], is a hypothetical 51.9 kDa protein. The human MPP1 protein is homologue of Nuf2p [P(N) of 1.3 × 10–10; high score of 183]. Note that MPP1 is a protein of 225 kDa (Fritzler et al., 2000). (B) The S.pombe homologue of Spc24p, C336.08, is a hypothetical 23.0 kDa protein [P(N) of 2.1 × 10–10; high score of 148]. Identical amino acids are highlighted in black and similar amino acids in grey.
Materials and methods
Growth media, strain and plasmid construction, and two-hybrid analysis
Basic yeast methods and growth media were as described previously (Sherman, 1991). Yeast strains were grown in yeast extract, peptone and dextrose medium containing 100 mg/l adenine (YPAD medium). Synthetic complete (SC) medium was used to select for plasmids in yeast. Yeast strains and plasmids are described in Table III. Yeast strains were constructed using PCR-amplified cassettes (Knop et al., 1999). To tag the CEN5 DNA, plasmid pXH136 (He et al., 2000) was restricted with BamHI and integrated next to CEN5 by homologous recombination. TetR–GFP of plasmid pXH123 (Michaelis et al., 1997) or pCJ092 was integrated into the LEU2 or ADE2 locus, respectively. For the construction of temperature-sensitive alleles, SPC24 and SPC25 were mutagenized by PCR and conditional lethal alleles were selected (Geissler et al., 1996). Two-hybrid interactions were analysed as described previously (Pereira et al., 1999).
Table III. Yeast strains and plasmids.
| Name | Genotype | Reference |
|---|---|---|
| CJY016 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1 SPC24-3HA::kanMX6 | this study |
| CJY045 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 Δspc25::HIS3MX4 leu2Δ1::pCJ020 | this study |
| CJY048 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 Δspc25::HIS3MX4 leu2Δ1::pCJ023 | this study |
| CJY083 | MATa Δspc24::kanMX6 ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1::pCJ062 | this study |
| CJY084 | MATa Δspc24::kanMX6 ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1::pCJ063 | this study |
| CJY092 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1 NDC80-6HA::klTRP1 | this study |
| CJY132 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 Δspc25::HIS3MX4 leu2Δ1::pCJ023 Δsst1::URA3 PDS1-6HA::klTRP1 | this study |
| CJY138 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1 Δsst1::URA3 PDS1-6HA::klTRP1 | this study |
| CJY176 | MATa Δspc24::kanMX6 ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1::pCJ062 Δsst1::URA3 PDS1-6HA::klTRP1 | this study |
| CJY184 | MATa ura3-52::pXH136 lys2-801 trp1Δ63 his3Δ200 leu2Δ1::pXH123 | this study |
| CJY189 | MATa ura3-52::pXH136 lys2-801 trp1Δ63 his3Δ200 leu2Δ1::pXH123 NUF2-9Myc::klTRP1 | this study |
| CJY191 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 Δspc25::HIS3MX4 leu2Δ1::pCJ023 Δsst1::URA3 MCM21-9Myc::kITRP1 | this study |
| CJY193 | MATa Δspc24::kanMX6 ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1::pCJ062 Δsst1::URA3 MCM21-9Myc::kITRP1 | this study |
| CJY195 | MATa ura3-52::pXH136 lys2-801 trp1Δ63 his3Δ200 leu2Δ1::pXH123 SPC98-9Myc::klTRP1 | this study |
| CJY197 | MATa ura3-52::pXH136 lys2-801 trp1Δ63 his3Δ200 leu2Δ1::pXH123 MCM21-9Myc::klTRP1 | this study |
| CJY231 | MATa ura3-52::pXH136 lys2-801 ade2-101::pCJ092 trp1Δ63 his3Δ200 Δspc25::HIS3MX4 leu2Δ1::pCJ023 | this study |
| CJY232 | MATa Δspc24::kanMX6 ura3-52::pXH136 lys2-801 ade2-101::pCJ092 trp1Δ63 his3Δ200 leu2Δ1::pCJ062 | this study |
| CJY233 | MATa Δmad2::kanMX6 ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1 PDS1-6HA::klTRP1 | this study |
| ESM480 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1 SPC25-3HA::kanMX6 | this study |
| ESM493 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1 SPC25-ProA::kanMX6 | this study |
| ESM835 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1 Δsst1 NUF2-6HA::kITRP1 | this study |
| ESM965 | MATa Δduo1::kanMX6 ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2::pSM808 PDS1-6HA-klTRP1 | this study |
| ESM1286 | MATa ura3-52 lys2-801 trp1Δ63 his3Δ200 leu2Δ1 ndc10-1 NUF2-GFP::kanMX6 | this study |
| ESM1287 | MATa ura3-52 lys2-801 trp1Δ63 his3Δ200 leu2Δ1 ndc10-1 SPC24-GFP::kanMX6 | this study |
| ESM1290 | MATa ura3-52 lys2-801 trp1Δ63 his3Δ200 leu2Δ1 ndc10-1 NDC80-6HA::klTRP1 | this study |
| ESM1291 | MATa ura3-52 lys2-801 trp1Δ63 his3Δ200 leu2Δ1 ndc10-1 NDC80-9Myc::klTRP1 | this study |
| ESM1292 | MATa ura3-52 lys2-801 trp1Δ63 his3Δ200 leu2Δ1 ndc10-1 SPC24-6HA::klTRP1 | this study |
| JK421 | MATa ura3-52 lys2-801 trp1Δ63 his3Δ200 leu2Δ1 ndc10-1 | Goh and Kilmartin (1993) |
| ndc80-1 | MATa ura3-52 lys2-801 trp1Δ63 his3Δ200 leu2Δ1 ndc80-1 | Wigge et al. (1998) |
| ndc80-2 | MATa ura3-52 lys2-801 trp1Δ63 his3Δ200 leu2Δ1 ndc80-2 | Wigge et al. (1998) |
| PSY455 | MATa leu2-3, 112 ura3-52 trp1Δ1 nuf2-61 | Osborne et al. (1994) |
| SGY37 | MATa ura3-52::URA3-lexA-op-LacZ trp1Δ63 his3Δ200 leu2Δ1 | Geissler et al. (1996) |
| YPH499 | MATa ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1 | Sikorski and Hieter (1989) |
| YPH500 | MATα ura3-52 lys2-801 ade2-101 trp1Δ63 his3Δ200 leu2Δ1 | Sikorski and Hieter (1989) |
| pACT2 | 2 µm, LEU2-based vector carrying the GAL4 activator domain | Durfee et al. (1993) |
| pCJ020 | spc25-4 in pRS305 | this study |
| pCJ023 | spc25-7 in pRS305 | this study |
| pCJ062 | spc24-2 in pRS305 | this study |
| pCJ063 | spc24-3 in pRS305 | this study |
| pCJ092 | tetR-GFP in pRS402 | this study |
| pEG202 | 2 µm, HIS3-based vector carrying the LexA DNA-binding domain | Gyuris et al. (1993) |
| pJO527 | MCM21 in pACT2 | Ortiz et al. (1999) |
| pRS305 | LEU2-based integration vector | Sikorski and Hieter (1989) |
| pRS316 | CEN6, URA3-based yeast–Escherichia coli shuttle vector | Sikorski and Hieter (1989) |
| pRS426 | 2 µm, URA3-based yeast–Escherichia coli shuttle vector | Christianson et al. (1992) |
| pSM149 | NUF2 (codon 1–177) in pGEX-4T-3 | this study |
| pSM150 | NUF2 (codon 244–452) in pGEX-4T-3 | this study |
| pSM539 | SPC25 in pACT2 | this study |
| pSM540 | SPC25 in pEG202 | this study |
| pSM542 | SPC24 in pACT2 | this study |
| pSM543 | SPC24 in pEG202 | this study |
| pSM557 | SPC34 in pACT2 | this study |
| pSM558 | SPC34 in pEG202 | this study |
| pSM561 | SPC25 in pRS316 | this study |
| pSM562 | SPC24 in pRS316 | this study |
| pSM702 | SPC24 in pGEX-5X-1 | this study |
| pSM742 | NUF2 in pRS426 | this study |
| pSM744 | SPC98 in pRS426 | this study |
| pSM746 | SPC24 in pRS426 | this study |
| pSM747 | SPC25 in pRS426 | this study |
| pSM758 | NUF2 (codon 1–177) in pACT2 | this study |
| pSM759 | NUF2 (codon 244–452) in pACT2 | this study |
| pSM764 | NUF2 (codon 1–177) in pEG202 | this study |
| pSM765 | NUF2 (codon 244–452) in pEG202 | this study |
| pSM778 | NDC80 in pACT2 | this study |
| pSM779 | NDC80 in pEG202 | this study |
| pSM791 | NDC80 in pRS426 | this study |
| pSM808 | duo1-2 in pRS305 | this study |
| pXH123 | tetR–GFP in a LEU2-based integration vector | Michaelis et al. (1997) |
| pXH136 | 112× tetO in pRS306-based integration vector containing recombination signal for chromosome V | He et al. (2000) |
Analysis of conditional lethal spc24 and spc25 mutants
Yeast cells were incubated at 23°C for 2.5 h with α-factor (1 µg/ml for Δsst1 and 10 µg/ml for SST1 cells) to arrest cells in G1 phase of the cell cycle. α-factor was removed by washing the cells with pre-warmed (37°C) YPAD medium (t = 0). Cells were then incubated in YPAD at 37°C. At the times indicated, cells were fixed with formaldehyde for indirect immunofluorescence, the budding index was determined after fixation in 70% ethanol and the DNA content was analysed by flow cytometry (Hutter and Eipel, 1979).
High gene dosage suppression and synthetic lethality
ndc80-1, ndc80-2, nuf2-61, spc24-2, spc24-3, spc25-4 and spc25-7 cells were transformed with NDC80, NUF2, SPC24, SPC25 and SPC98 on the 2 µm, high-copy-number plasmid pRS426 and with pRS426. Transformants were tested for 3–4 days for growth at 23, 30, 33, 35 and 37°C. To test for synthetic lethality, ndc80-1, ndc80-2, nuf2-61, spc24-2, spc24-3, spc25-4 and spc25-7 cells were complemented with the defective gene on pRS316. MCM21 was disrupted using a PCR-based cassette containing the TRP1 marker from Kluyveromyces lactis (klTRP1) (Knop et al., 1999). Disruption was confirmed by colony PCR. Δmcm21 cells were grown on 5-fluoroorotic acid (5-FOA) plates at 23, 30, 33, 35 and 37°C for 3 days.
Purification of Spc25p–ProA
SPC25–ProA cells and SPC25 control cells were grown to mid-logarithmic phase in YPAD medium at 30°C. The cell pellet (5 g each) was resuspended in IP-buffer (50 mM Tris–HCl pH 7.6, 10 mM EDTA, 1 mM EGTA, 100 mM NaCl, 5% glycerol) containing protease inhibitors [cØmplete™ (from Boehringer Mannheim), 1 mM phenylmethylsulfonyl fluoride, 4 µg/ml pepstatin, 2.5 mM benzamidine]. After cell breakage, 1% Triton X-100 was added and cells were incubated at 4°C for 45 min. The cleared lysate (10 000 g for 15 min at 4°C) was incubated with magnetic beads coated with rabbit IgGs (1.5 h). The beads were washed with IP-buffer, 1% Triton X-100 and IP-buffer alone. Proteins were eluted from the beads by heating the beads in sample buffer (Knop et al., 1999) for 15 min at 65°C. The protein samples were resolved by a 6–18% SDS–PAGE gradient gel and stained with Coomassie Blue. Selected protein bands were analysed by MALDI analysis (Shevchenko et al., 1996).
ChIP
ChIPs of yeast cells were performed as described previously (Hecht and Grunstein, 1999). The anti-HA antibody (3F10) was used for the immunoprecipitation of HA-tagged proteins at a concentration of 0.6 ng/ml. The primers used to amplify the CEN DNA and the non-target DNAs are described (Ortiz et al., 1999) as CENwt, III-L and III-R. The ChIP PCR reactions contained 0.025% of the chromatin preparation for the input and 1.8% of the chromatin preparation for the immunoprecipitations.
Immunological techniques and microscopy
Anti-N-Nuf2p, anti-C-Nuf2p and anti-Spc24p antibodies were raised in rabbits against purified glutathione S-transferase (GST) fusion proteins. Antibodies were affinity purified using GST fusion proteins coupled to CNBr–Sepharose (Pharmacia).
The polyclonal rabbit anti-Mcm21p, anti-Okp1p (Ortiz et al., 1999), anti-Tub4p (Spang et al., 1996), anti-Spc72p (Knop and Schiebel, 1998) and anti-Spc110p antibodies (Knop and Schiebel, 1997) have been described. Rabbit polyclonal or mouse monoclonal anti-β-tubulin antibody (Wa3) was used to detect Tub2p (Spang et al., 1996). Mouse monoclonal anti-HA antibodies (12CA5 or 3F10) or anti-Myc antibody (9E10) were obtained from Hiss Diagnostics, Boehringer Mannheim or Boehringer Ingelheim. Secondary antibodies used were goat anti-mouse and goat anti-rabbit antibodies coupled to Cy2, Cy3 or 6-((7-amino- 4-methylcoumarin-3-acetyl)amino)hexanoic acid (AMCA), or goat anti-rabbit antibodies coupled to horseradish peroxidase (Jackson ImmunoResearch Laboratories). Immunofluorescence of formaldehyde-fixed yeast cells was performed with 40 min fixation time. Samples were analysed using either a Zeiss Axiophot microscope or a Leica SP2 LCS confocal microscope.
Immunoprecipitation of Ndc80p-6HA and Spc25p-6HA proteins was performed as described (Pereira et al., 1998) using IP-buffer (see above). The anti-HA antibodies were cross-linked to protein A–Sepharose beads (Pereira et al., 1999). Proteins were separated by SDS–PAGE with broad range molecular weight markers from Bio-Rad. Immunoreactions were visualized by an enhanced chemiluminescence (ECL) kit from Amersham.
Acknowledgments
Acknowledgements
The work of E.S. was supported by the HFSP (RG0319/1999) and the Cancer Research Campaign. We thank K.Nasmyth, J.Kilmartin, P.Silver and P.Sorger for antibodies, plasmids or yeast strains. We are grateful to G.Pereira and J.Grindlay for carefully reading the manuscript, and to R.H.Wilson and K.Vass for advice on sequence analysis.
REFERENCES
- Abrieu A., Kahana,J.A., Wood,K.W. and Cleveland,D.W. (2000) CENP-E as an essential component of the mitotic checkpoint in vitro. Cell, 102, 817–826. [DOI] [PubMed] [Google Scholar]
- Altschul S.F. and Gish,W. (1996) Local alignment statistics. Methods Enzymol., 266, 460–480. [DOI] [PubMed] [Google Scholar]
- Bardin A.J., Visintin,R. and Amon,A. (2000) A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell, 102, 21–31. [DOI] [PubMed] [Google Scholar]
- Chan G.K., Jablonski,S.A., Sudakin,V., Hittle,J.C. and Yen,T.J. (1999) Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol., 146, 941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.H., Waters,J.C., Salmon,E.D. and Murray,A.W. (1996) Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science, 274, 242–246. [DOI] [PubMed] [Google Scholar]
- Chen Y., Riley,D.J., Chen,P.-L. and Lee,W.-H. (1997) HEC, a novel nuclear protein rich in leucine heptad repeats specifically involved in mitosis. Mol. Cell. Biol., 17, 6049–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho R.J., Fromont-Racine,M., Wodicka,L., Feierbach,B., Stearns,T., Legrain,P., Lockhart,D.J. and Davis,R.W. (1998) Parallel analysis of genetic selections using whole genome oligonucleotide arrays. Proc. Natl Acad. Sci. USA, 95, 3752–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T.W., Sikorski,R.S., Dante,M., Shero,J.H. and Hieter,P. (1992) Multifunctional yeast high-copy-number shuttle vectors. Gene, 110, 119–122. [DOI] [PubMed] [Google Scholar]
- Donaldson D. and Kilmartin,V. (1996) Spc42p: a phosphorylated component of the S. cerevisiae spindle pole body (SPB) with an essential function during SPB duplication. J. Cell Biol., 132, 887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee T., Becherer,K., Chen,P.L., Yeh,S.H., Yang,Y., Kilburn,A.E., Lee,W.H. and Elledge,S.J. (1993) The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev., 7, 555–569. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Hayes M., Clarke,L. and Carbon,J. (1982) Nucleotide sequence comparisons and functional analysis of yeast centromere DNAs. Cell, 29, 235–244. [DOI] [PubMed] [Google Scholar]
- Fritzler M.J., Kerfoot,S.M., Feasby,T.E., Zochodne,D.W., Westendorf,J.M., Dalmau,J.O. and Chan,E.K. (2000) Autoantibodies from patients with idiopathic ataxia bind to M-phase phosphoprotein-1 (MPP1). J. Invest. Med., 48, 28–39. [PubMed] [Google Scholar]
- Geissler S., Pereira,G., Spang,A., Knop,M., Souès,S., Kilmartin,J. and Schiebel,E. (1996) The spindle pole body component Spc98p interacts with the γ-tubulin-like Tub4p of Saccharomyces cerevisiae at the sites of microtubule attachment. EMBO J., 15, 3899–3911. [PMC free article] [PubMed] [Google Scholar]
- Goh P.Y. and Kilmartin,J.V. (1993) NDC10: a gene involved in chromosome segregation in Saccharomyces cerevisiae. J. Cell Biol., 121, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G. and Yanagida,M. (2000) Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell, 100, 619–633. [DOI] [PubMed] [Google Scholar]
- Gyuris J., Golemis,E., Chertkov,H. and Brent,R. (1993) Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell, 75, 791–803. [DOI] [PubMed] [Google Scholar]
- He X., Asthana,S. and Sorger,P.K. (2000) Transient sister chromatid separation and elastic deformation of chromosomes during mitosis in budding yeast. Cell, 101, 763–775. [DOI] [PubMed] [Google Scholar]
- Hecht A. and Grunstein,M. (1999) Mapping DNA interaction sites of chromosomal proteins using immunoprecipitation and polymerase chain reaction. Methods Enzymol., 304, 399–414. [DOI] [PubMed] [Google Scholar]
- Hofmann C., Cheeseman,I.M., Goode,B.L., McDonald,K.L., Barnes,G. and Drubin,D.G. (1998) Saccharomyces cerevisiae Duo1p and Dam1p, novel proteins involved in mitotic spindle function. J. Cell Biol., 143, 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter K.J. and Eipel,H.E. (1979) Microbial determination by flow cytometry. J. Gen. Microbiol., 113, 369–375. [DOI] [PubMed] [Google Scholar]
- Hwang L.H., Lau,L.F., Smith,D.L., Mistrot,C.A., Hardwick,K.G., Hwang,E.S., Amon,A. and Murray,A.W. (1998) Budding yeast Cdc20: a target of the spindle checkpoint. Science, 279, 1041–1044. [DOI] [PubMed] [Google Scholar]
- Hyland K.M., Kingsbury,J., Koshland,D. and Hieter,P. (1999) Ctf10p: a novel kinetochore protein in Saccharomyces cerevisiae and a potential link between the kinetochore and mitotic spindle. J. Cell Biol., 145, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q.-W., Fuchs,J. and Loidl,J. (2000) Centromere clustering is a major determinant of yeast interphase nuclear organization. J. Cell Sci., 113, 1903–1912. [DOI] [PubMed] [Google Scholar]
- Kilmartin J.V., Dyos,S.L., Kershaw,D. and Finch,J.T. (1993) A spacer protein in the Saccharomyces cerevisiae spindle pole body whose transcription is cell-cycle regulated. J. Cell Biol., 123, 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M. and Schiebel,E. (1997) Spc98p and Spc97p of the yeast γ-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J., 16, 6985–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M. and Schiebel,E. (1998) Receptors determine the cellular localization of a γ-tubulin complex and thereby the site of microtubule formation. EMBO J., 17, 3952–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M., Siegers,K., Pereira,G., Zachariae,W., Winsor,B., Nasmyth,K. and Schiebel,E. (1999) Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast, 15, 963–972. [DOI] [PubMed] [Google Scholar]
- Lechner J. (1994) A zinc-finger protein, essential for chromosome segregation, constitutes a putative DNA-binding subunit of the Saccharomyces cerevisiae kinetochore complex, CBF3. EMBO J., 13, 5203–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner J. and Carbon,J. (1991) A 240kd multisubunit protein complex, CBF3, is a major component of the budding yeast centromere. Cell, 64, 717–725. [DOI] [PubMed] [Google Scholar]
- Meluh P.B. and Koshland,D. (1995) Evidence that the MIF2 gene of Saccharomyces cerevisiae encodes a centromere protein with homology to the mammalian centromere protein CENP-C. Mol. Biol. Cell, 6, 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meluh P.B., Yang,P., Glowczewski,L., Koshland,D. and Smith,M.M. (1998) Cse4p is a component of the core centromere of Saccharomyces cerevisiae. Cell, 94, 607–613. [DOI] [PubMed] [Google Scholar]
- Michaelis C., Ciosk,R. and Nasmyth,K. (1997) Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell, 91, 35–45. [DOI] [PubMed] [Google Scholar]
- Neff N.F., Thomas,J.H., Grisafi,P. and Botstein,D. (1983) Isolation of the β-tubulin gene from yeast and demonstration of its essential function in vivo. Cell, 33, 211–219. [DOI] [PubMed] [Google Scholar]
- Newman J.R.S., Wolf,E. and Kim,P.S. (2000) A computationally directed screen identifying interacting coiled coils from Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 97, 13203–12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J., Stemmann,O., Rank,S. and Lechner,J. (1999) A putative protein complex consisting of Ctf19, Mcm21, and Okp1 represents a missing link in the budding yeast kinetochore. Genes Dev., 13, 1140–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne M.A., Schlenstedt,G., Jinks,T. and Silver,P.A. (1994) Nuf2, a spindle pole body-associated protein required for nuclear division in yeast. J. Cell Biol., 125, 853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G., Knop,M. and Schiebel,E. (1998) Spc98p directs the yeast γ-tubulin complex into the nucleus and is subject to cell cycle-dependent phosphorylation on the nuclear side of the spindle pole body. Mol. Biol. Cell, 9, 775–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G., Grueneberg,U., Knop,M. and Schiebel,E. (1999) Interaction of the yeast γ-tubulin complex binding protein Spc72p with Kar1p is essential for microtubule function during karyogamy. EMBO J., 18, 4180–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G., Höfken,T., Grindlay,J., Manson,C. and Schiebel,E. (2000) The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol. Cell, 6, 1–10. [PubMed] [Google Scholar]
- Pidoux A.L. and Allshire,R.C. (2000) Centromeres: getting a grip of chromosomes. Curr. Opin. Cell Biol., 12, 308–319. [DOI] [PubMed] [Google Scholar]
- Rout M.P. and Kilmartin,J.V. (1990) Components of the yeast spindle and spindle pole body. J. Cell Biol., 111, 1913–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. (1991) Getting started with yeast. Methods Enzymol., 194, 3–21. [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Jensen,O.N., Podtelejnikov,A.V., Sagliocco,F., Wilm,M., Vorm,O., Mortensen,P., Boucherie,H. and Mann,M. (1996) Linking genome and proteome by mass spectrometry: large scale identification of yeast proteins from two dimensional gels. Proc. Natl Acad. Sci. USA, 93, 14440–14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R.S. and Hieter,P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae.Genetics, 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A., Geissler,S., Grein,K. and Schiebel,E. (1996) γ-tubulin-like Tub4p of Saccharomyces cerevisiae is associated with the spindle pole body substructures that organize microtubules and is required for mitotic spindle formation. J. Cell Biol., 134, 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer F. and Hieter,P. (1992) Centromere DNA mutations induce a mitotic delay in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 89, 8908–8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmann O. and Lechner,J. (1996) The Saccharomyces cerevisiae kinetochore contains a cyclin–cdk complex homologue, as identified by in-vitro reconstitution. EMBO J., 15, 3611–3620. [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Murakami,S., Chikashige,Y., Funabiki,H., Niwa,O. and Yanagida,M. (1992) A low copy number central sequence with strict symmetry and unusual chromatin structure in fission yeast centromere. Mol. Biol. Cell, 3, 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Fuchs,J., Loidl,J. and Nasmyth,K. (2000) Cohesin ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation. Nature Cell Biol., 2, 492–499. [DOI] [PubMed] [Google Scholar]
- Taylor S.S. and McKeon,F. (1997) Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell, 89, 727–735. [DOI] [PubMed] [Google Scholar]
- Taylor S.S., Ha,E. and McKeon,F. (1998) The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub3-related protein kinase. J. Cell Biol., 142, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Gibson,T.J., Plewniak,F., Jeanmougin,F. and Higgins,D.G. (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res., 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F., Wenic,D., Pouparet,M.-A., Koonin,E.V. and Nasmyth,K. (2000) Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell, 103, 375–386. [DOI] [PubMed] [Google Scholar]
- Wang Y.C. and Burke,D.J. (1995) Checkpoint genes required to delay cell division in response to nocodazole respond to impaired kinetochore function in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol., 15, 6838–6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf J.M., Rao,P.N. and Gerace,L. (1994) Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc. Natl Acad. Sci. USA, 91, 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P.A., Jensen,O.N., Holmes,S., Souès,S., Mann,M. and Kilmartin,J.V. (1998) Analysis of the Saccharomyces spindle pole by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. J. Cell Biol., 141, 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Guacci,V. and Koshland,D. (1996) Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s). J. Cell Biol., 133, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Chen,Y. and Lee,W.-H. (1999) Hec1p, an evolutionary conserved coiled-coil protein, modulates chromosome segregation through interaction with SMC proteins. Mol. Cell. Biol., 19, 5417–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]