Abstract
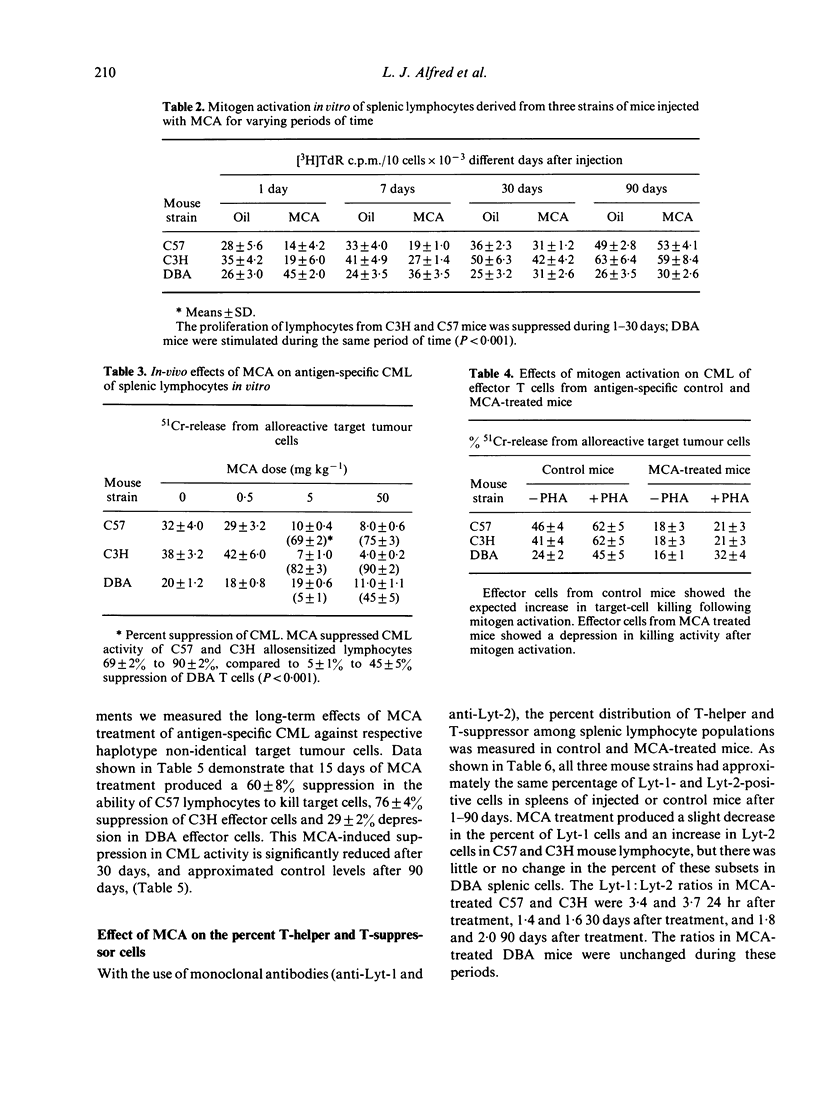
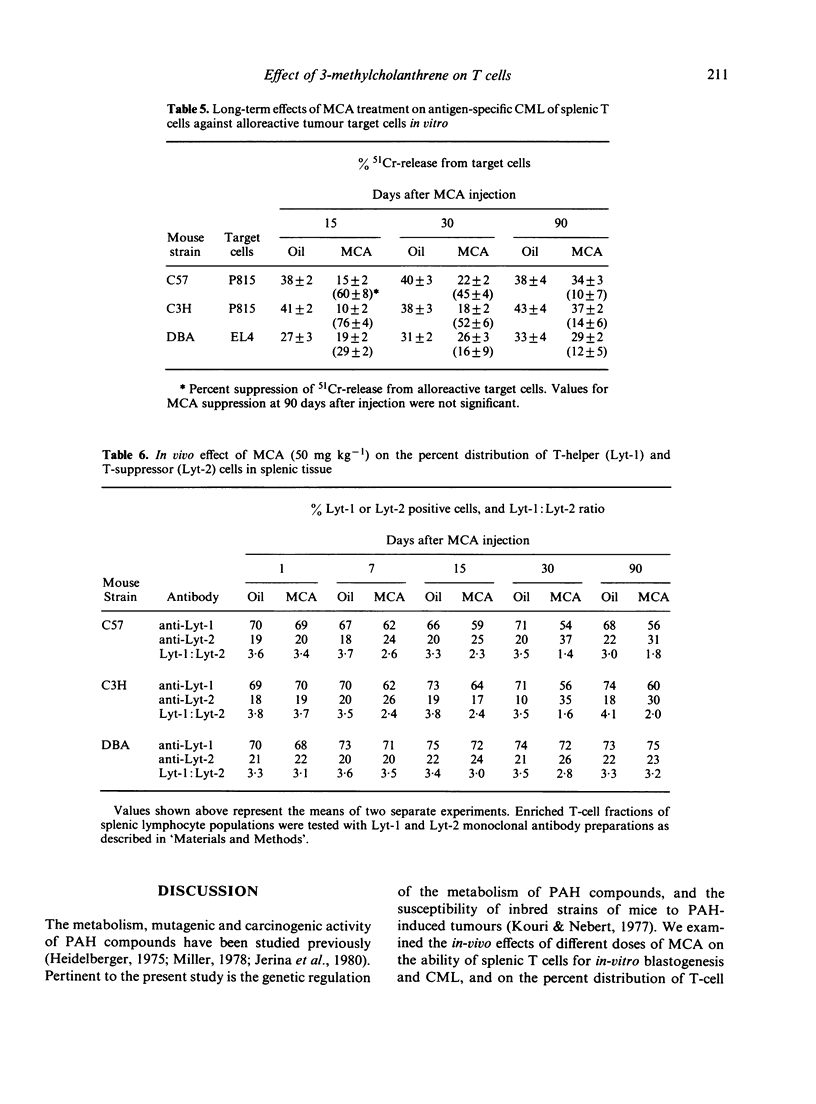
The in-vivo effects of a polycyclic aromatic hydrocarbon (PAH), 3-methylcholanthrene (MCA), on in-vitro mitogen activation, cell-mediated lympholysis (CML) and T-cell subset distribution in mouse splenic lymphocyte populations were measured. Three inbred mouse strains were treated with a single intraperitoneal injection of corn oil alone or with different doses of MCA in oil (0.5-50 mg kg -1). One to ninety days after injection, splenic lymphocytes were isolated, and assayed for blastogenesis, CML and the percent T-helper and T-suppressor cells using monoclonal antibodies. High doses of MCA suppressed mitogen activation (15.2-53.6%) and CML (69-90%) within 24 hr in lymphocytes from PAH-responsive mice (C57 and C3H). Blastogenesis was stimulated and CML was suppressed to a lesser degree (5-45%) in lymphocytes from non-responsive mice (DBA). MCA induced an increase in T-suppressor cells in responsive mice, but there was no change in DBA mice. These studies suggest a correlation between immunocytotoxicity of PAH compounds on T-cell subsets and the responsiveness of mouse strains to these carcinogens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfred L. J., Wojdani A. Effects of methylcholanthrene and benzanthracene on blastogenesis and aryl hydrocarbon hydroxylase induction in splenic lymphocytes from three inbred strains of mice. Int J Immunopharmacol. 1983;5(2):123–129. doi: 10.1016/0192-0561(83)90003-6. [DOI] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Ernster L. The metabolism of polycyclic hydrocarbons and its relationship to cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):149–186. doi: 10.1016/0304-419x(78)90013-6. [DOI] [PubMed] [Google Scholar]
- Gehly E. B., Landolph J. R., Heidelberger C., Nagasawa H., Little J. B. Induction of cytotoxicity, mutation, cytogenetic changes, and neoplastic transformation by benzo(a)pyrene and derivatives in C3H/10T1/2 clone 8 mouse fibroblasts. Cancer Res. 1982 May;42(5):1866–1875. [PubMed] [Google Scholar]
- Haubeck H. D., Kölsch E. Regulation of immune responses aginst the syngeneic ADJ-PC-5 plasmacytoma in BALB-c mice. III. Induction of specific T suppressor cells to the BALB/c plasmacytoma ADJ-PC-5 during early stages of tumorigenesis. Immunology. 1982 Nov;47(3):503–510. [PMC free article] [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Prehn R. T. Immunostimulation of chemical oncogenesis in the mouse. Int J Cancer. 1977 Dec 15;20(6):918–922. doi: 10.1002/ijc.2910200615. [DOI] [PubMed] [Google Scholar]
- Sims P., Grover P. L. Epoxides in polycyclic aromatic hydrocarbon metabolism and carcinogenesis. Adv Cancer Res. 1974;20:165–274. doi: 10.1016/s0065-230x(08)60111-6. [DOI] [PubMed] [Google Scholar]


