Abstract
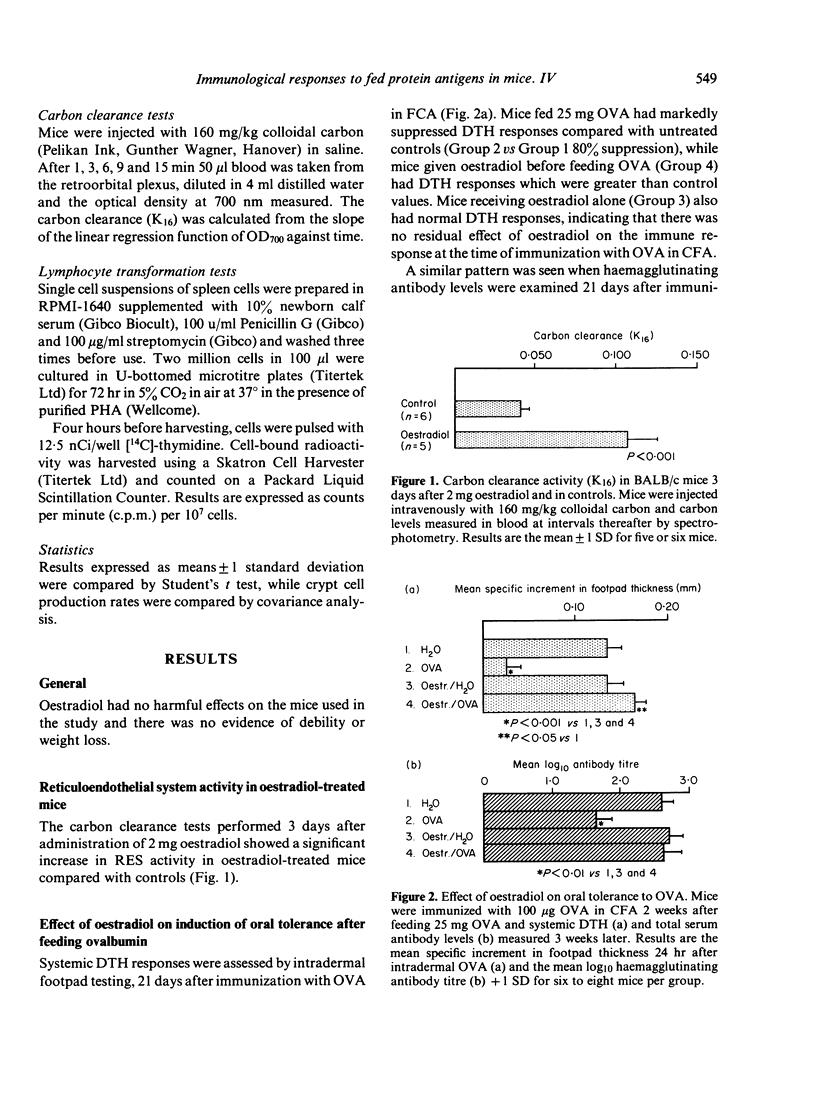
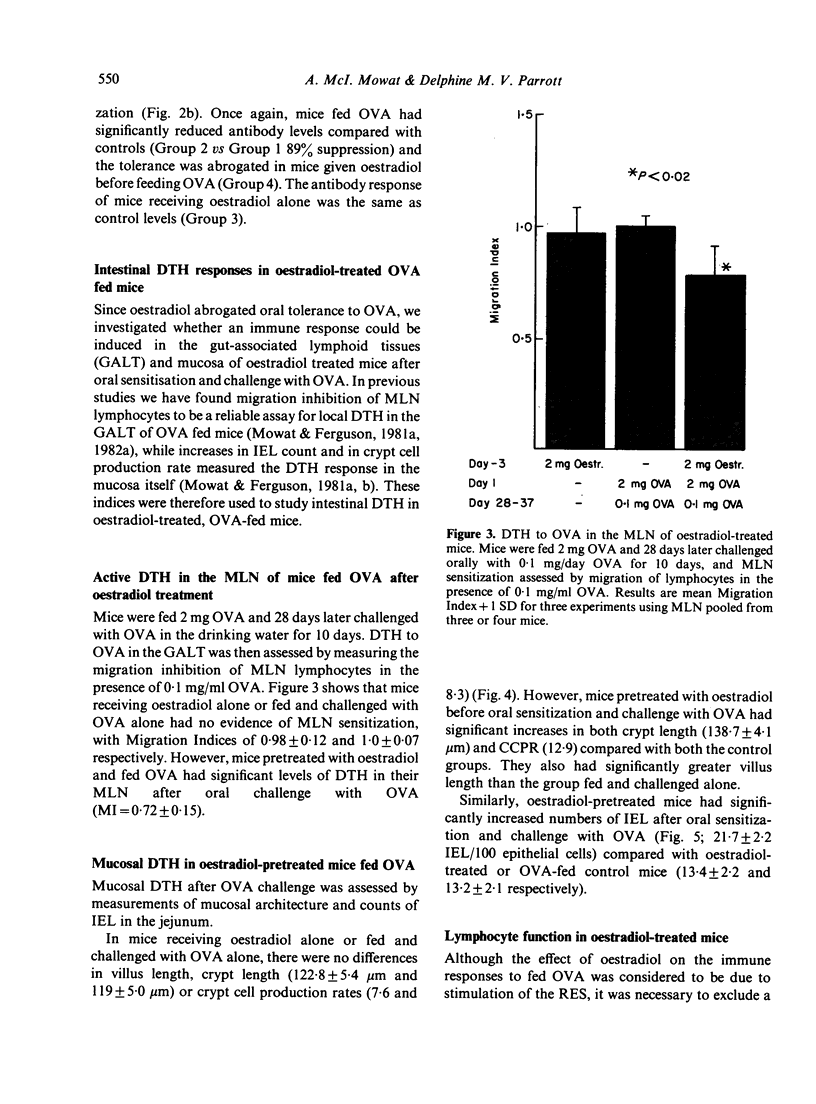
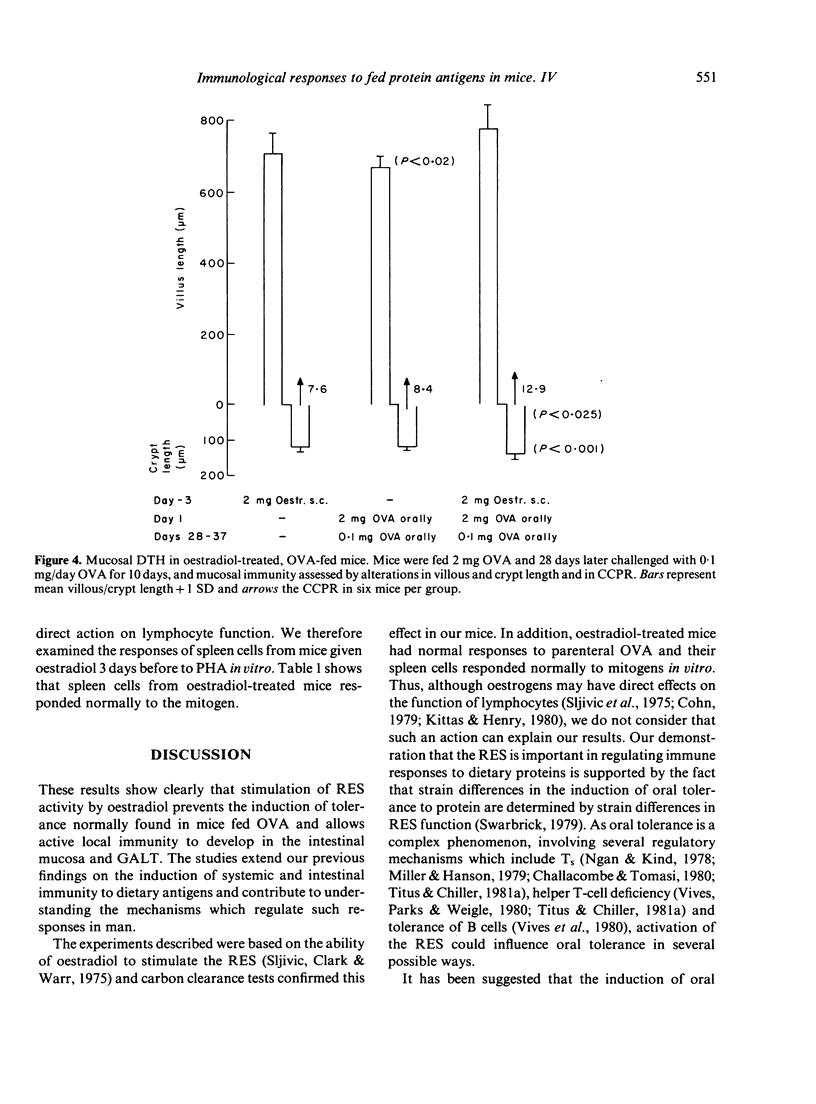
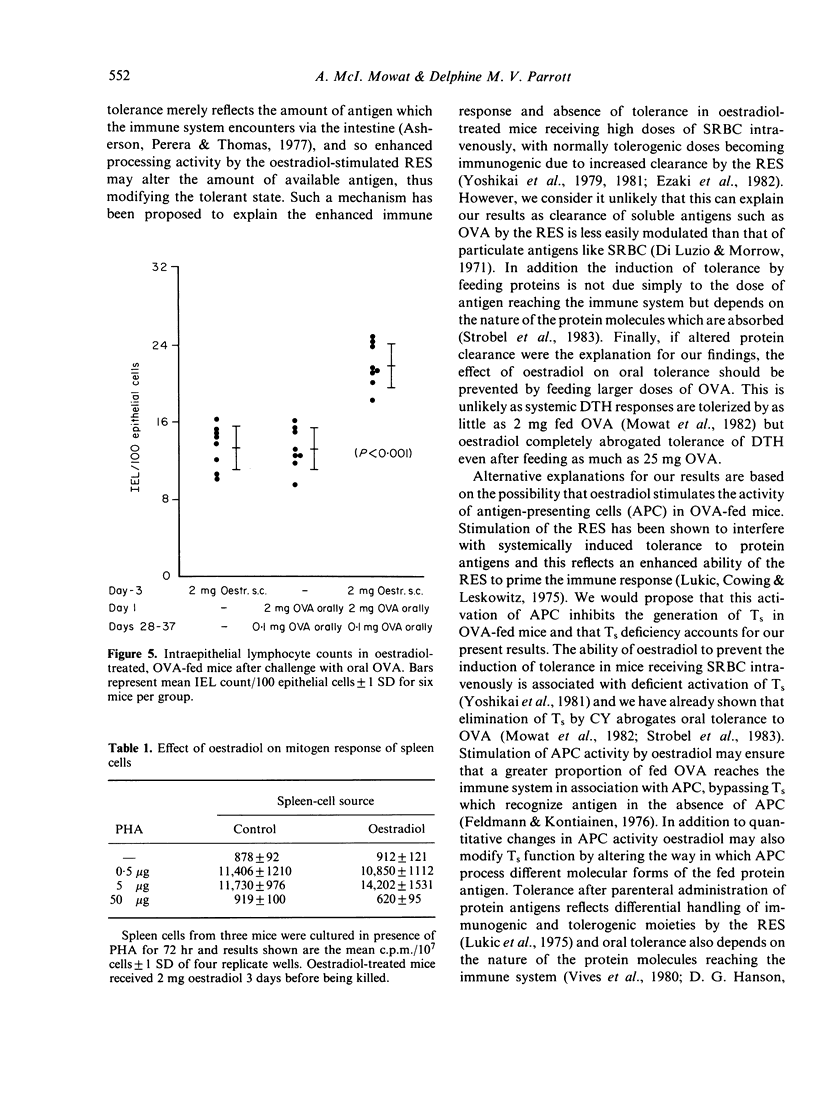
We have studied the role of the reticuloendothelial system (RES) in intestinal and systemic immunity in mice immunized orally with ovalbumin (OVA). Stimulation of the RES by oestradiol completely prevented the induction of systemic tolerance normally found in mice fed 25 mg OVA and this applied both to humoral immunity and delayed-type hypersensitivity (DTH). In addition, an active DTH response could be detected in the mucosa and mesenteric lymph nodes (MLN) of oestradiol-treated, OVA-fed mice on oral challenge with OVA. Oestradiol had no direct effect on lymphocyte function and we propose that RES activation may be one mechanism which predisposes to small intestinal disease associated with food hypersensitivity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Perera M. A., Thomas W. R. Contact sensitivity and the DNA response in mice to high and low doses of oxazolone: low dose unresponsiveness following painting and feeding and its prevention by pretreatment with cyclophosphamide. Immunology. 1979 Mar;36(3):449–459. [PMC free article] [PubMed] [Google Scholar]
- Challacombe S. J., Tomasi T. B., Jr Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980 Dec 1;152(6):1459–1472. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. A. High sensitivity to androgen as a contributing factor in sex differences in the immune response. Arthritis Rheum. 1979 Nov;22(11):1218–1233. doi: 10.1002/art.1780221109. [DOI] [PubMed] [Google Scholar]
- Di Luzio N. R., Morrow H. S., 3rd Comparative behavior of soluble and particulate antigens and inert colloids in reticuloendothelial-stimulated or depressed mice. J Reticuloendothel Soc. 1971 Mar;9(3):273–287. [PubMed] [Google Scholar]
- Ezaki T., Nawa Y., Hayama T., Yamaguchi K., Kotani M. Modulation of the immune responses against SRBC after oestriol treatment in mice. Clin Exp Immunol. 1982 Apr;48(1):239–243. [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Kontiainen S. Suppressor cell induction in vitro. II. Cellular requirements of suppressor cell induction. Eur J Immunol. 1976 Apr;6(4):302–305. doi: 10.1002/eji.1830060413. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971 Dec;12(12):988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittas C., Henry L. Effect of sex hormones on the response of mice to infection with Toxoplasma gondii. Br J Exp Pathol. 1980 Dec;61(6):590–600. [PMC free article] [PubMed] [Google Scholar]
- Lukić M. L., Cowing C., Leskowitz S. Strain differences in ease of tolerance induction to bovine gamma-globulin: dependence on macrophage function. J Immunol. 1975 Jan;114(1 Pt 2):503–506. [PubMed] [Google Scholar]
- Miller S. D., Hanson D. G. Inhibition of specific immune responses by feeding protein antigens. IV. Evidence for tolerance and specific active suppression of cell-mediated immune responses to ovalbumin. J Immunol. 1979 Nov;123(5):2344–2350. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Hypersensitivity in the small intestinal mucosa. V. Induction of cell-mediated immunity to a dietary antigen. Clin Exp Immunol. 1981 Mar;43(3):574–582. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Migration inhibition of lymph node lymphocytes as an assay for regional cell-mediated immunity in the intestinal lymphoid tissues of mice immunized orally with ovalbumin. Immunology. 1982 Oct;47(2):365–370. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Migration inhibition of lymph node lymphocytes as an in vitro assay for cell-mediated immunity in the draining lymph nodes of parenterally immunized mice. Immunology. 1982 Oct;47(2):357–364. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Strobel S., Drummond H. E., Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982 Jan;45(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Newby T. J., Stokes C. R., Bourne F. J. Altered polyvinylpyrrolidone clearance and immune responsiveness caused by small dietary changes. Clin Exp Immunol. 1980 Feb;39(2):349–354. [PMC free article] [PubMed] [Google Scholar]
- Ngan J., Kind L. S. Suppressor T cells for IgE and IgG in Peyer's patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978 Mar;120(3):861–865. [PubMed] [Google Scholar]
- Sljivić V. S., Clark D. W., Warr G. W. Effects of oestrogens and pregnancy on the distribution of sheep erythrocytes and the antibody response in mice. Clin Exp Immunol. 1975 Apr;20(1):179–186. [PMC free article] [PubMed] [Google Scholar]
- Staszak C., Goodwin J. S., Troup G. M., Pathak D. R., Williams R. C., Jr Decreased sensitivity to prostaglandin and histamine in lymphocytes from normal HLA-B12 individuals: a possible role in autoimmunity. J Immunol. 1980 Jul;125(1):181–185. [PubMed] [Google Scholar]
- Strobel S., Mowat A. M., Drummond H. E., Pickering M. G., Ferguson A. Immunological responses to fed protein antigens in mice. II. Oral tolerance for CMI is due to activation of cyclophosphamide-sensitive cells by gut-processed antigen. Immunology. 1983 Jul;49(3):451–456. [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Chiller J. M. Orally induced tolerance. Definition at the cellular level. Int Arch Allergy Appl Immunol. 1981;65(3):323–338. [PubMed] [Google Scholar]
- Tomasi T. B., Jr Oral tolerance. Transplantation. 1980 May;29(5):353–356. doi: 10.1097/00007890-198005000-00001. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Miake S., Matsumoto T., Nomoto K., Takeya K. Effect of stimulation and blockade of mononuclear phagocyte system on the delayed footpad reaction to SRBC in mice. Immunology. 1979 Nov;38(3):577–583. [PMC free article] [PubMed] [Google Scholar]
- Yoshikai Y., Miake S., Matsumoto T., Nomoto K., Takeya K. Effect of stimulation and blockade of mononuclear phagocyte system on the induction of suppressor T cells of delayed footpad reaction to SRBC in mice. Immunology. 1981 Jun;43(2):241–247. [PMC free article] [PubMed] [Google Scholar]


