Abstract
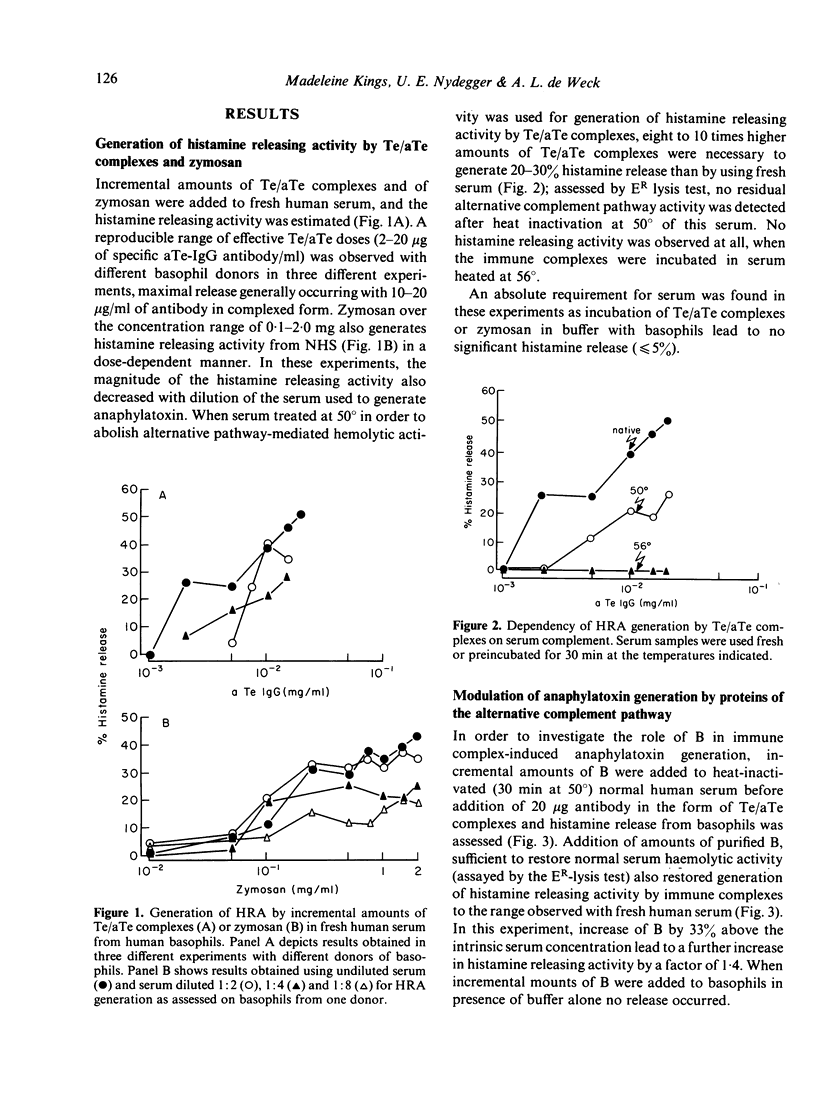
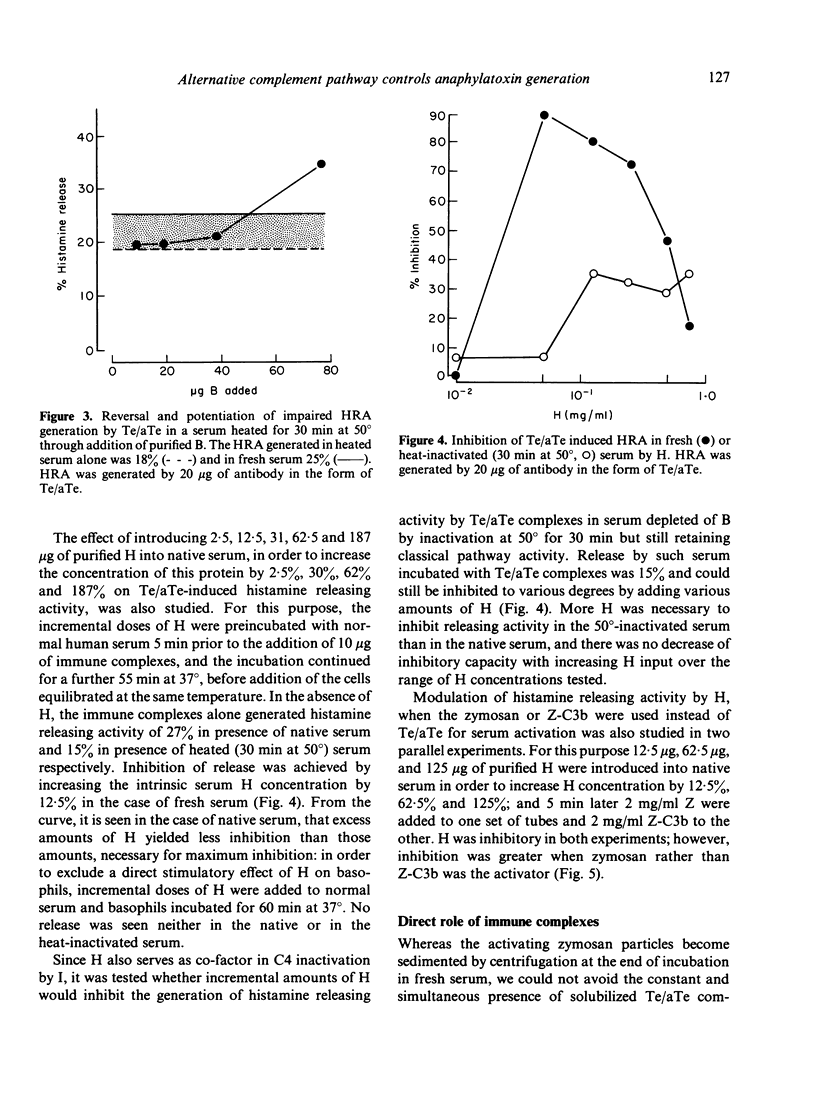
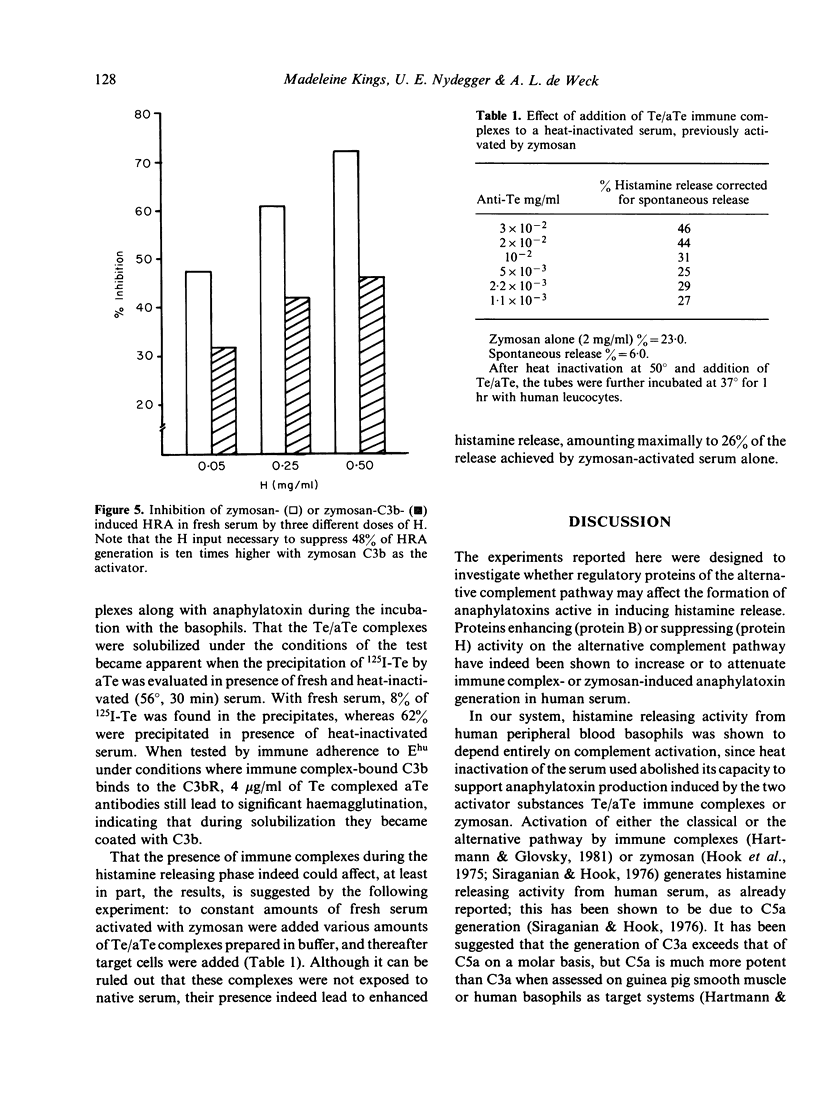
The generation of histamine releasing activity (HRA) from human basophils in fresh serum by tetanus toxoid (Te)/anti-Te complexes or by zymosan can be modulated through introduction of incremental amounts of proteins B and H of the alternative complement pathway. Serum treated at 50 degrees in order to abolish alternative pathway-mediated haemolytic activity, lost 90% of its capacity to generate HRA upon addition of Te/aTe; such loss could be reversed through additions of purified B. Amounts of B sufficient to restore normal alternative pathway haemolytic activity also restored HRA induced by Te/aTe; as little as a 33% increase above the normal serum concentration of B increased the capacity to support Te/aTe induced HRA by a factor of 1.4. In contrast, additions of incremental doses of purified H to fresh serum reduced generation of HRA by both Te/aTe and zymosan. Total inhibition was achieved by increasing the serum H concentration by 12.5-30%; further increases of H up to 200% again permitted HRA generation induced by immune complexed aTe. H also inhibited Te/aTe induced HRA in a serum heated at 50 degrees but only 30% inhibition of HRA could be achieved over a range of H inputs up to 187% above normal serum concentration. Additions of H also inhibited HRA generation in fresh serum when induced with plain or C3b-coated zymosan (Z) particles. By increasing the serum concentration of H from 12.5 to 125%, dose-dependent inhibition of HRA generation was observed; the H input necessary to suppress 48% of HRA generation was ten times higher when HRA was generated by Z-C3b than by plain zymosan. Thus, the complement-dependent generation of HRA from fresh serum strongly depends on modest variations in the concentrations of the two regulatory proteins B and H of the alternative complement pathway, suggesting their direct effect on generation of anaphylatoxins C3a and C5a.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chenoweth D. E., Goodman M. G., Weigle W. O. Demonstration of a specific receptor for human C5a anaphylatoxin on murine macrophages. J Exp Med. 1982 Jul 1;156(1):68–78. doi: 10.1084/jem.156.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daha M. R., Fearon D. T., Austen K. F. C3 requirements for formation of alternative pathway C5 convertase. J Immunol. 1976 Aug;117(2):630–634. [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Formation of a hemolytically active cellular intermediate by the interaction between properdin factors B and D and the activated third component of complement. J Exp Med. 1973 Dec 1;138(6):1305–1313. doi: 10.1084/jem.138.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. The physiological breakdown of the third component of human complement. Mol Immunol. 1980 Jan;17(1):9–20. doi: 10.1016/0161-5890(80)90119-4. [DOI] [PubMed] [Google Scholar]
- Hartman C. T., Jr, Glovsky M. M. Complement activation requirements for histamine release from human leukocytes: influence of purified C3ahu and C5ahu on histamine release. Int Arch Allergy Appl Immunol. 1981;66(3):274–281. doi: 10.1159/000232831. [DOI] [PubMed] [Google Scholar]
- Hernandez-Asensio M., Hooks J. J., Ida S., Siraganian R. P., Notkins A. L. Interferon-induced enhancement of IgE-mediated histamine release from human basophils requires RNA synthesis. J Immunol. 1979 Apr;122(4):1601–1603. [PubMed] [Google Scholar]
- Hook W. A., Siraganian R. P., Wahl S. M. Complement-induced histamine release from human basophils. I. Generation of activity in human serum. J Immunol. 1975 Apr;114(4):1185–1190. [PubMed] [Google Scholar]
- Hunsicker L. G., Ruddy S., Austen K. F. Alternate complement pathway: factors involved in cobra venom factor (CoVF) activation of the third component of complement (C3). J Immunol. 1973 Jan;110(1):128–138. [PubMed] [Google Scholar]
- Johnson A. R., Hugli T. E., Müller-Eberhard H. J. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975 Jun;28(6):1067–1080. [PMC free article] [PubMed] [Google Scholar]
- Kazatchkine M. D., Fearon D. T., Austen K. F. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J Immunol. 1979 Jan;122(1):75–81. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miyakawa Y., Yamada A., Kosaka K., Tsuda F., Kosugi E., Mayumi M. Defective immune-adherence (C3b) receptor on erythrocytes from patients with systemic lupus erythematosus. Lancet. 1981 Sep 5;2(8245):493–497. doi: 10.1016/s0140-6736(81)90882-5. [DOI] [PubMed] [Google Scholar]
- Needleman B. W., Weiler J. M., Feldbush T. L. The third component of complement inhibits human lymphocyte blastogenesis. J Immunol. 1981 Apr;126(4):1586–1591. [PubMed] [Google Scholar]
- Nicol P. A., Lachmann P. J. The alternate pathway of complement activation. The role of C3 and its inactivator (KAF). Immunology. 1973 Feb;24(2):259–275. [PMC free article] [PubMed] [Google Scholar]
- Nomenclature of the alternative activating pathway of complement. J Immunol. 1981 Sep;127(3):1261–1262. [PubMed] [Google Scholar]
- Nydegger U. E., Corvetta A., Spaeth P. J., Spycher M. The alternative complement pathway control protein H binds to immune complexes and serves their detection. Clin Immunol Immunopathol. 1983 Jan;26(1):56–65. doi: 10.1016/0090-1229(83)90173-3. [DOI] [PubMed] [Google Scholar]
- Nydegger U. E., Fearon D. T., Austen K. F. The modulation of the alternative pathway of complement in C2-deficient human serum by changes in concentration of the component and control proteins. J Immunol. 1978 Apr;120(4):1404–1408. [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf R. E., Hammann K. P., Scheiner O., Lemmel E. M., Dierich M. P. Activation of human monocytes by both human beta 1H and C3b. Immunology. 1982 Jun;46(2):307–312. [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Glovsky M. M., Ward P. A. C3a-induced lysosomal enzyme secretion from human neutrophils: lack of inhibition by f met-leu-phe antagonists and inhibition by arachidonic acid antagonists. Int Arch Allergy Appl Immunol. 1982;67(3):227–232. doi: 10.1159/000233023. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P., Hook W. A. Complement-induced histamine release from human basophils. II. Mechanism of the histamine release reaction. J Immunol. 1976 Mar;116(3):639–646. [PubMed] [Google Scholar]
- Siraganian R. P. Refinements in the automated fluorometric histamine analysis system. J Immunol Methods. 1975 Jun;7(2-3):283–290. doi: 10.1016/0022-1759(75)90025-3. [DOI] [PubMed] [Google Scholar]
- Thomas L. L., Findlay S. R., Lichtenstein L. M. Augmentation of antigen-stimulated histamine release from human basophils by serum-treated zymosan particles. II. Dependence on IgE-mediated release. J Immunol. 1979 Oct;123(4):1468–1472. [PubMed] [Google Scholar]
- Thomas L. L., Lichtenstein L. M. Augmentation of antigen-stimulated histamine release from human basophils by serum-treated zymosan particles. I. Characteristics of enhancement. J Immunol. 1979 Oct;123(4):1462–1467. [PubMed] [Google Scholar]
- Thompson R. A., Winterborn M. H. Hypocomplementaemia due to a genetic deficiency of beta 1H globulin. Clin Exp Immunol. 1981 Oct;46(1):110–119. [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Nguyen B. Y., Sisson S. P., Kim Y. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol. 1982 Oct;129(4):1681–1687. [PubMed] [Google Scholar]
- Vogt W., Schmidt G., Lynen R., Dieminger L. Cleavage of the third complement component (C3) and generation of the spasmogenic peptide, C3a, in human serum via the properdin pathway: demonstration of inhibitory as well as enhancing effects of epsilon-amino-caproic acid. J Immunol. 1975 Feb;114(2 Pt 1):671–677. [PubMed] [Google Scholar]
- Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]


