Abstract
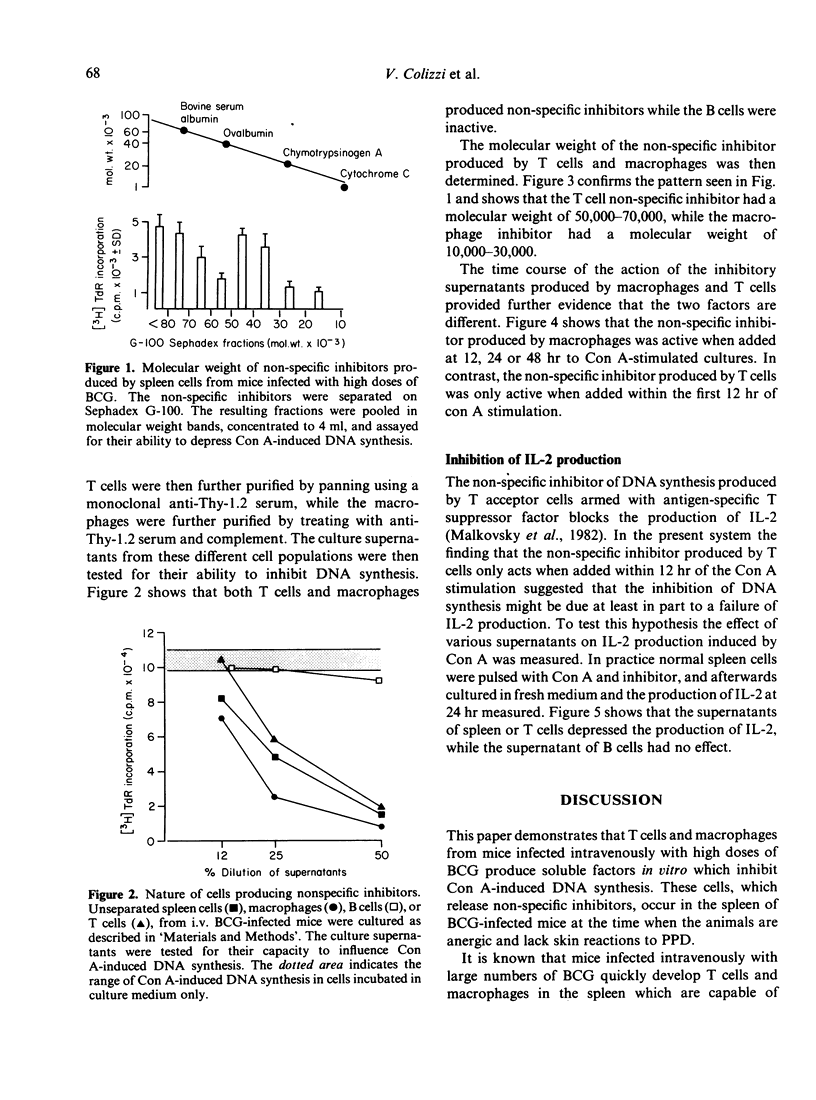
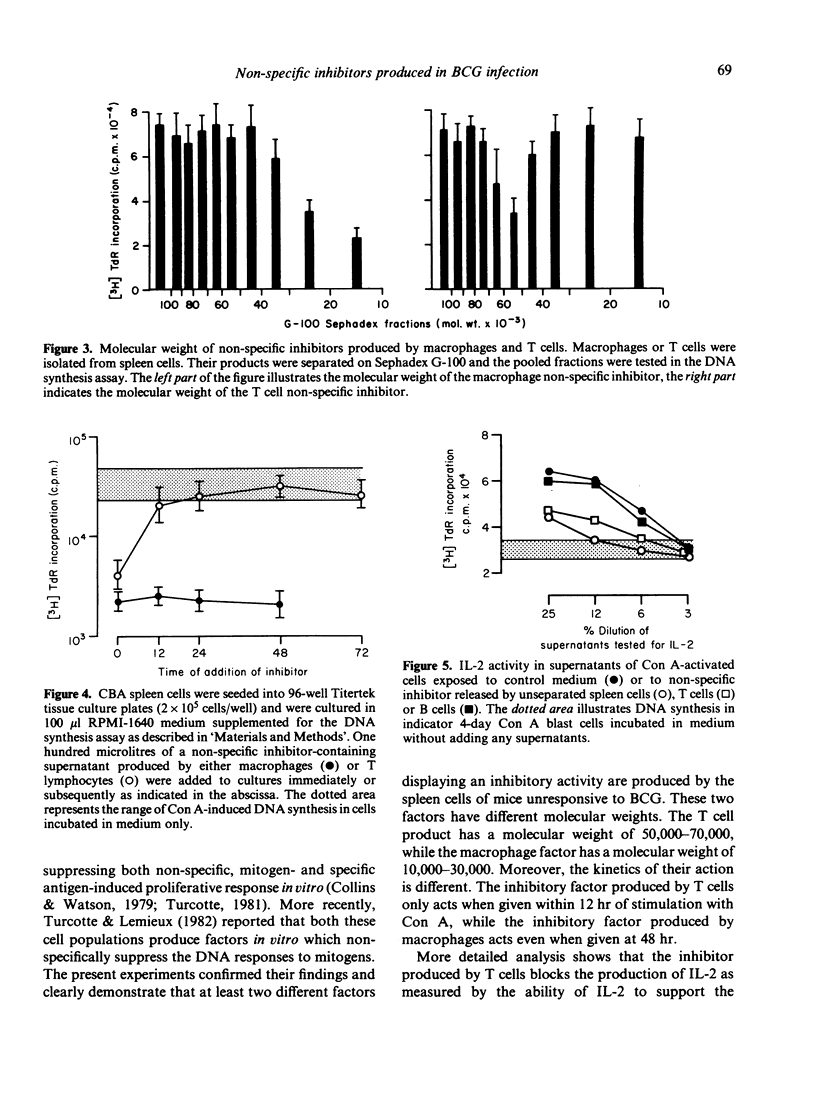
Mice injected intravenously with a high dose (5 X 10(7) ) of BCG fail to develop delayed hypersensitivity to BCG and are described as anergic or unresponsive. Spleen cells from these mice release factors on culture which suppress DNA synthesis induced by concanavalin A in vitro. Cell separation experiments showed that both macrophages and T cells produce inhibitory factors. However, the macrophage factor has a molecular weight 10,000-30,000, while the T cell factor has a molecular weight of 50,000-70,000. Further evidence that these two factors are different is provided by the kinetics of their action. The T cell factor only acts when given within 12 hr of stimulation with concanavalin A, while the macrophage factor acts even when given at 48 hr. In the case of the T cell factor, the inhibition of DNA synthesis may be attributed to its ability to block the interleukin-2 production induced by Con A. As similar T cell and macrophage factors are produced in mice responding to simple chemically reactive haptenes (contact sensitizers), it is possible that a similar suppressor circuit is involved in the control of the response to contact sensitizers and in the production of unresponsiveness (anergy) in mice given large doses of BCG.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Zembala M. The role of the T acceptor cell in suppressor systems. Antigen-specific T suppressor factor acts via a T acceptor cell; this releases a nonspecific inhibitor of the transfer of contact sensitivity when exposed to antigen in the context of I-J. Ann N Y Acad Sci. 1982;392:71–89. doi: 10.1111/j.1749-6632.1982.tb36099.x. [DOI] [PubMed] [Google Scholar]
- Bullock W. E. Anergy and infection. Adv Intern Med. 1976;21:149–173. [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentin I., Orbach-Arbouys S. Réactivité des cellules spléniques après traitement par le BCG: variation de la réponse selon les tests et les legnées utilisées. Ann Immunol (Paris) 1977 Jan-Mar;128(1-2):271–272. [PubMed] [Google Scholar]
- Granelli-Piperno A., Vassalli J. D., Reich E. Purification of murine T cell growth factor. A lymphocyte mitogen with helper activity. J Exp Med. 1981 Aug 1;154(2):422–431. doi: 10.1084/jem.154.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Deficit of interleukin 2 production associated with impaired T-cell proliferative responses in Mycobacterium lepraemurium infection. Infect Immun. 1983 Jan;39(1):109–116. doi: 10.1128/iai.39.1.109-116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoureux G., Poisson R. Letter: B.C.G. and immunological anergy. Lancet. 1974 May 18;1(7864):989–990. doi: 10.1016/s0140-6736(74)91296-3. [DOI] [PubMed] [Google Scholar]
- Malkovsky M., Asherson G. L., Stockinger B., Watkins M. C. Nonspecific inhibitor released by T acceptor cells reduces the production of interleukin-2. Nature. 1982 Dec 16;300(5893):652–655. doi: 10.1038/300652a0. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Tokunaga T. Induction of suppressor T cells in delayed-type hypersensitivity to Mycobacterium bovis BCG in low-responder mice. Infect Immun. 1980 May;28(2):331–335. doi: 10.1128/iai.28.2.331-335.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Zembala M., Asherson G. L., Marcinkiewicz J. Inhibition of contact sensitivity by macrophages. Int Arch Allergy Appl Immunol. 1981;65(2):121–128. doi: 10.1159/000232747. [DOI] [PubMed] [Google Scholar]
- Ptak W., Zembala M., Hanczakowski-Rewicka M., Asherson G. L. Nonspecific macrophage suppressor factor: its role in the inhibition of contact sensitivity to picryl chloride by specific T suppressor factor. Eur J Immunol. 1978 Sep;8(9):645–649. doi: 10.1002/eji.1830080908. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Miller S. D., Moorhead J. W., Claman H. N. Active suppression of 1-fluoro-2,4-dinitrobenzene-immune T cells. Requirement of an auxiliary T cell induced by antigen. J Exp Med. 1979 May 1;149(5):1197–1207. doi: 10.1084/jem.149.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Evidence for two distinct populations of suppressor cells in the spleens of Mycobacterium bovis BCG-Sensitized mice. Infect Immun. 1981 Nov;34(2):315–322. doi: 10.1128/iai.34.2.315-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R., Lemieux S. Mechanisms of action of Mycobacterium bovis BCG-induced suppressor cells in mitogen-induced blastogenesis. Infect Immun. 1982 Apr;36(1):263–270. doi: 10.1128/iai.36.1.263-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembala M. A., Asherson G. L., James B. M., Stein V. E., Watkins M. C. Anti-haptene T suppressor factor acts through an I-J+, Ly1-2+, T acceptor cell that releases a nonspecific inhibitor of the transfer of contact sensitivity when exposed to antigen. J Immunol. 1982 Nov;129(5):1823–1829. [PubMed] [Google Scholar]


