Abstract
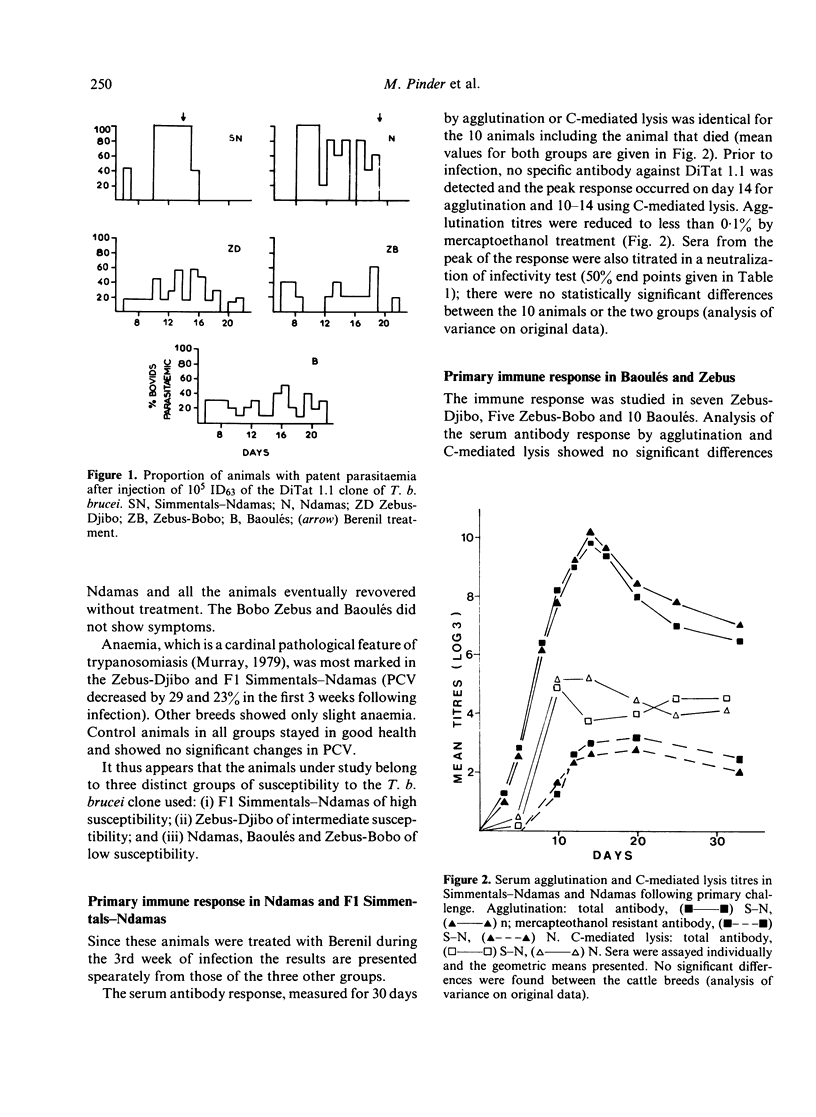
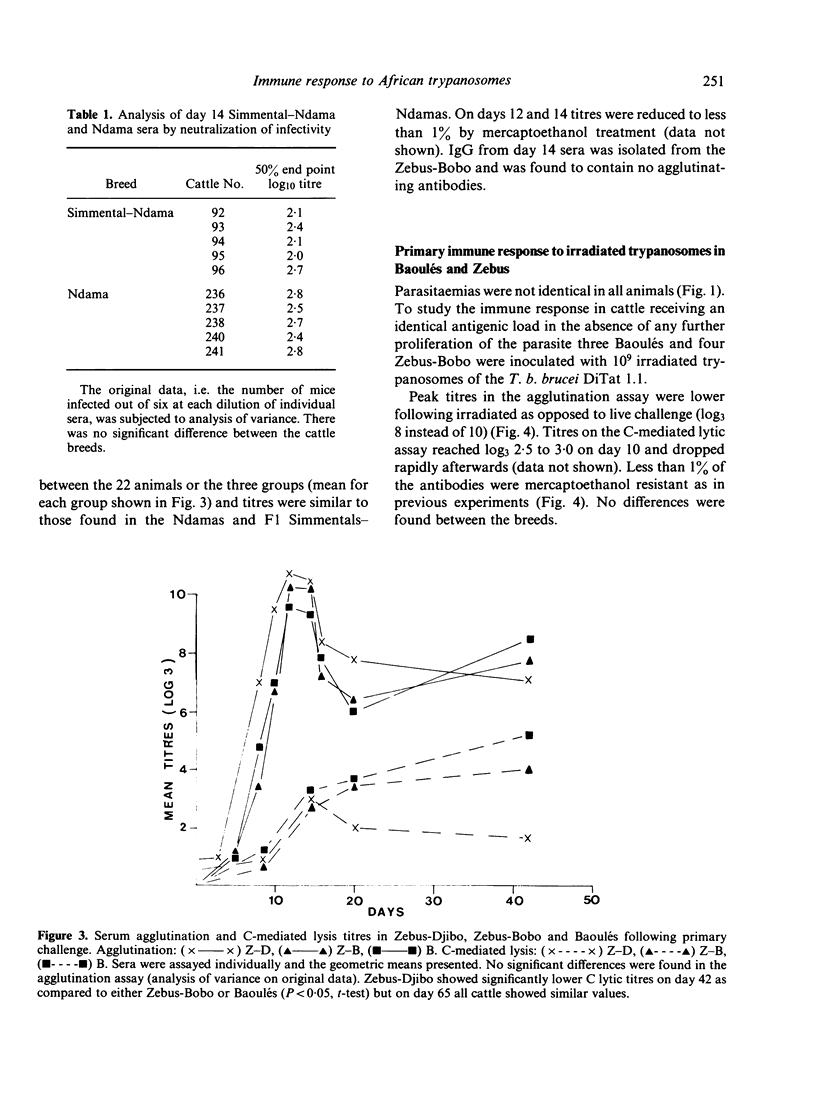
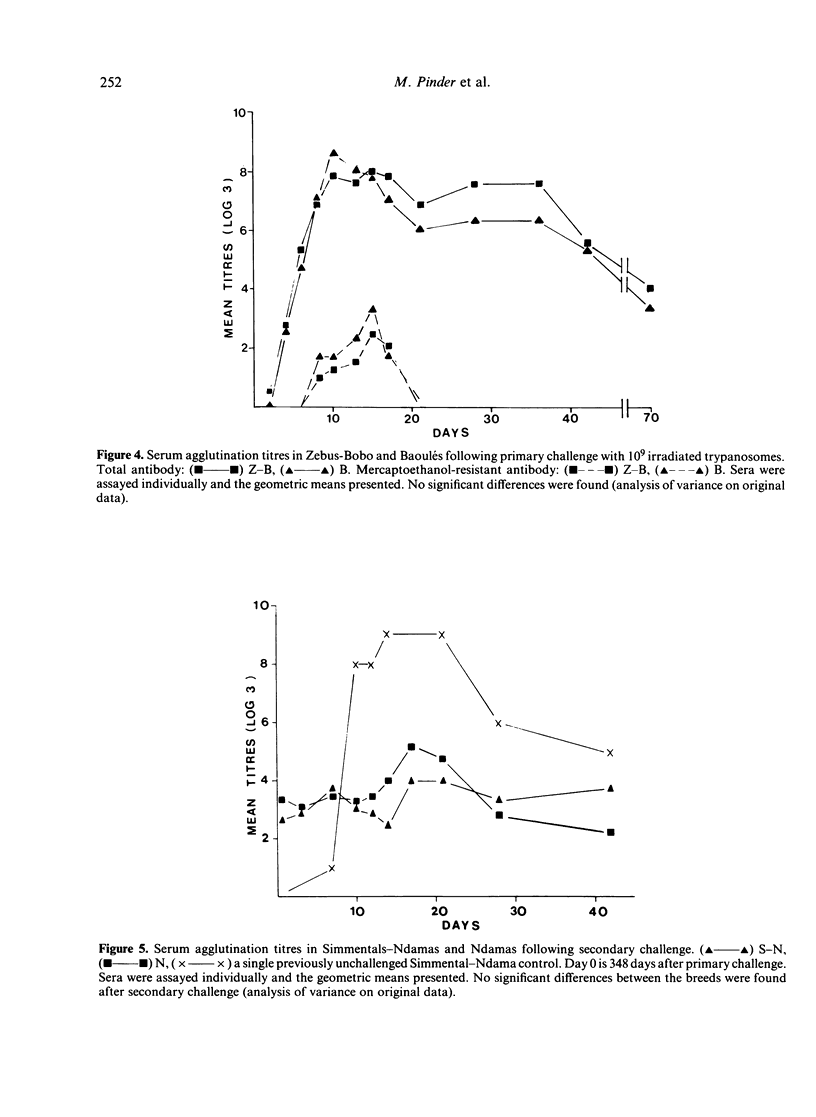
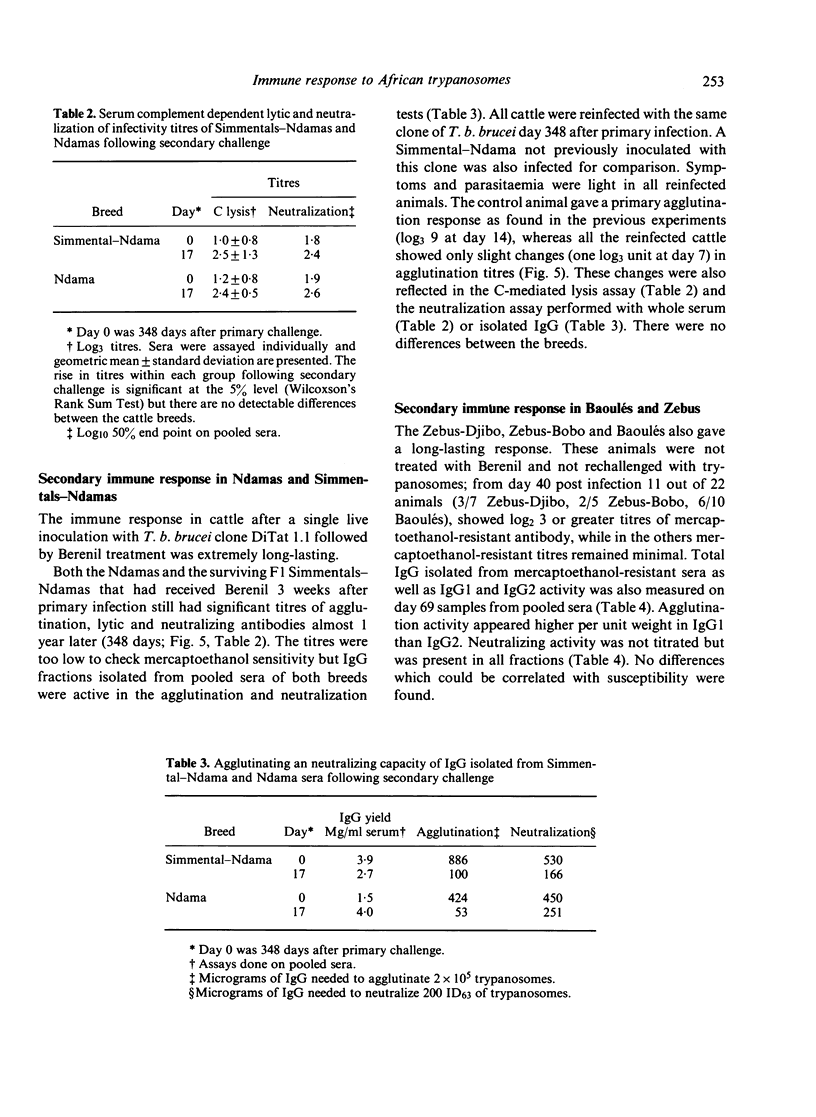
A clone of Trypanosoma brucei brucei (DiTat 1.1) was injected into 32 bovids of various breeds, 11 animals being kept as controls. Five animals, Simmental-Ndama F1 crosses, were extremely sensitive. They showed overt symptoms and one died on day 18 of infection despite treatment with a trypanocidal drug. Seven other animals became ill but recovered progressively and cleared the infection. Twenty animals, of breeds generally considered to be trypanosensitive as well as ones from trypanotolerant breeds, did not show symptoms apart from anaemia and cleared the infection. Putative protective antibody, i.e. directed against exposed determinants on the coat variant-specific glycoprotein, was detected by agglutination, complement-mediated lysis and inhibition of infectivity. All animals showed a primary immune response consisting of IgM whose kinetics and amplitude were indistinguishable between animals of differing sensitivity. The response was long-lasting, whether the animals had been treated or not with a trypanocidal drug 3 weeks after infection, and antibody of IgG1 and IgG2 types were detected in certain sensitive as well as resistant animals after 2 months. Some animals were rechallenged with DiTat 1.1 either 1 year after the primary infection or 6 months after inoculation of irradiated trypanosomes. Peak titres of antibody were lower than was the case following primary infection but higher levels of mercaptoenthanol-resistant antibodies were seen. In no case was there any difference in the response of sensitive or tolerant animals. Our results do not support the idea that resistance of certain bovids to African trypanosomiasis is due to a better protective antibody response.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell G. H., Phillips S. M. Adoptive transfer of variant-specific resistance to Trypanosoma rhodesiense with B lymphocytes and serum. Infect Immun. 1976 Nov;14(5):1144–1150. doi: 10.1128/iai.14.5.1144-1150.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESOWITZ R. S. Studies on immunity and host-parasite relationships. I. The immunological response of resistant and susceptible breeds of cattle to trypanosomal challenge. Ann Trop Med Parasitol. 1959 Sep;53:293–313. [PubMed] [Google Scholar]
- Gray A. R. A pattern in the development of agglutinogenic antigens of cyclically transmitted isolates of Trypanosoma gambiense. Trans R Soc Trop Med Hyg. 1975;69(1):131–138. doi: 10.1016/0035-9203(75)90022-x. [DOI] [PubMed] [Google Scholar]
- Guidot G., Roelants G. E. Sensibilité de Taurins Baoulé et de Zébus à Trypanosoma (Duttonella) vivax et T. (Nannomonas) congolense. Rev Elev Med Vet Pays Trop. 1982;35(3):233–244. [PubMed] [Google Scholar]
- Herbert W. J., Parratt D., Van Meirvenne N., Lennox B. An accidental laboratory infection with trypanosomes of a defined stock. II. Studies on the serological response of the patient and the identity of the infecting organism. J Infect. 1980 Jun;2(2):113–124. doi: 10.1016/s0163-4453(80)91109-3. [DOI] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Luckins A. G. The immune response of zebu cattle infection with Trypanosoma congolense and T. vivax. Ann Trop Med Parasitol. 1976 Jun;70(2):133–145. doi: 10.1080/00034983.1976.11687107. [DOI] [PubMed] [Google Scholar]
- Mansfield J. M., Levine R. F., Dempsey W. L., Wellhausen S. R., Hansen C. T. Lymphocyte function in experimental African trypanosomiasis. IV. Immunosuppression and suppressor cells in the athymic nu/nu mouse. Cell Immunol. 1981 Sep 1;63(1):210–215. doi: 10.1016/0008-8749(81)90043-5. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Rajasekariah G. R., Rickard M. D. A mechanism to account for mouse strain variation in resistance to the larval cestode, Taenia taeniaeformis. Immunology. 1980 Apr;39(4):481–489. [PMC free article] [PubMed] [Google Scholar]
- Murray M., Morrison W. I., Whitelaw D. D. Host susceptibility to African trypanosomiasis: trypanotolerance. Adv Parasitol. 1982;21:1–68. doi: 10.1016/s0065-308x(08)60274-2. [DOI] [PubMed] [Google Scholar]
- Murray M., Murray P. K., McIntyre W. I. An improved parasitological technique for the diagnosis of African trypanosomiasis. Trans R Soc Trop Med Hyg. 1977;71(4):325–326. doi: 10.1016/0035-9203(77)90110-9. [DOI] [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Barbet A. F., Kironde F., McGuire T. C. Bovine immune response to African ;trypanosomes: specific antibodies to variable surface glycoproteins of Trypanosoma brucei. Parasite Immunol. 1981 Summer;3(2):97–106. doi: 10.1111/j.1365-3024.1981.tb00388.x. [DOI] [PubMed] [Google Scholar]
- Nantulya V. M., Musoke A. J., Barbet A. F., Roelants G. E. Evidence for reappearance of Trypanosoma brucei variable antigen types in relapse populations. J Parasitol. 1979 Oct;65(5):673–679. [PubMed] [Google Scholar]
- Oka M., Osaki H., Furuya M., Ito Y., Oka Y. Agglutination antibody responses to Trypanosoma gambiense homogenate in mice treated with dextran sulfate 500 and carrageenan. Zentralbl Bakteriol Mikrobiol Hyg A. 1981;250(1-2):173–181. [PubMed] [Google Scholar]
- Roelants G. E., Pinder M. Immunobiology of African trypanosomiasis. Contemp Top Immunobiol. 1984;12:225–274. doi: 10.1007/978-1-4684-4571-8_7. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Askonas B. A. Trypanosome-induced suppression of anti-parasite responses during experimental African trypanosomiasis. Eur J Immunol. 1980 Dec;10(12):971–974. doi: 10.1002/eji.1830101216. [DOI] [PubMed] [Google Scholar]
- Seed J. R., Cornille R. L., Risby E. L., Gam A. A. The presence of agglutinating antibody in the IgM immunoglobulin fraction of rabbit antiserum during experimental African trypanosomiasis. Parasitology. 1969 May;59(2):283–292. doi: 10.1017/s003118200008224x. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Murray M. African trypanosome antigens recognized during the course of infection in N'dama and Zebu cattle. Infect Immun. 1982 Feb;35(2):410–416. doi: 10.1128/iai.35.2.410-416.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi T., Enriquez G. L. Effects of the IgG and IgM immunoglobulins in Trypanosoma gambiense infections in mice. J Parasitol. 1973 Aug;59(4):644–647. [PubMed] [Google Scholar]
- Turner M. J. Biochemistry of the variant surface glycoproteins of salivarian trypanosomes. Adv Parasitol. 1982;21:69–153. doi: 10.1016/s0065-308x(08)60275-4. [DOI] [PubMed] [Google Scholar]
- Wellde B. T., Duxbury R. E., Sadun E. H., Langbehn H. R., Lötzsch R., Deindl G., Warui G. Experimental infections with African trypanosomes. IV. Immunization of cattle with gamma-irradiated Trypanosoma rhodesiense. Exp Parasitol. 1973 Aug;34(1):62–68. doi: 10.1016/0014-4894(73)90063-5. [DOI] [PubMed] [Google Scholar]
- Wiesenhütter E. Versuche zur Immunisierung von Zeburindern gegen eine Trypanosoma-congolense-Infektion. Berl Munch Tierarztl Wochenschr. 1970 Jun 1;83(11):218–220. [PubMed] [Google Scholar]
- Wilson A. J., Cunningham M. P. Immunological aspects of bovine trypanosomiasis. I. Immune response of cattle to infection with Trypanosoma congolense and the antigenic variation of the infecting organisms. Exp Parasitol. 1972 Aug;32(1):165–173. doi: 10.1016/0014-4894(72)90021-5. [DOI] [PubMed] [Google Scholar]
- Zahalsky A. C., Weinberg R. L. Immunity to monomorphic Trypanosoma brucei: humoral response. J Parasitol. 1976 Feb;62(1):15–19. [PubMed] [Google Scholar]


