Abstract
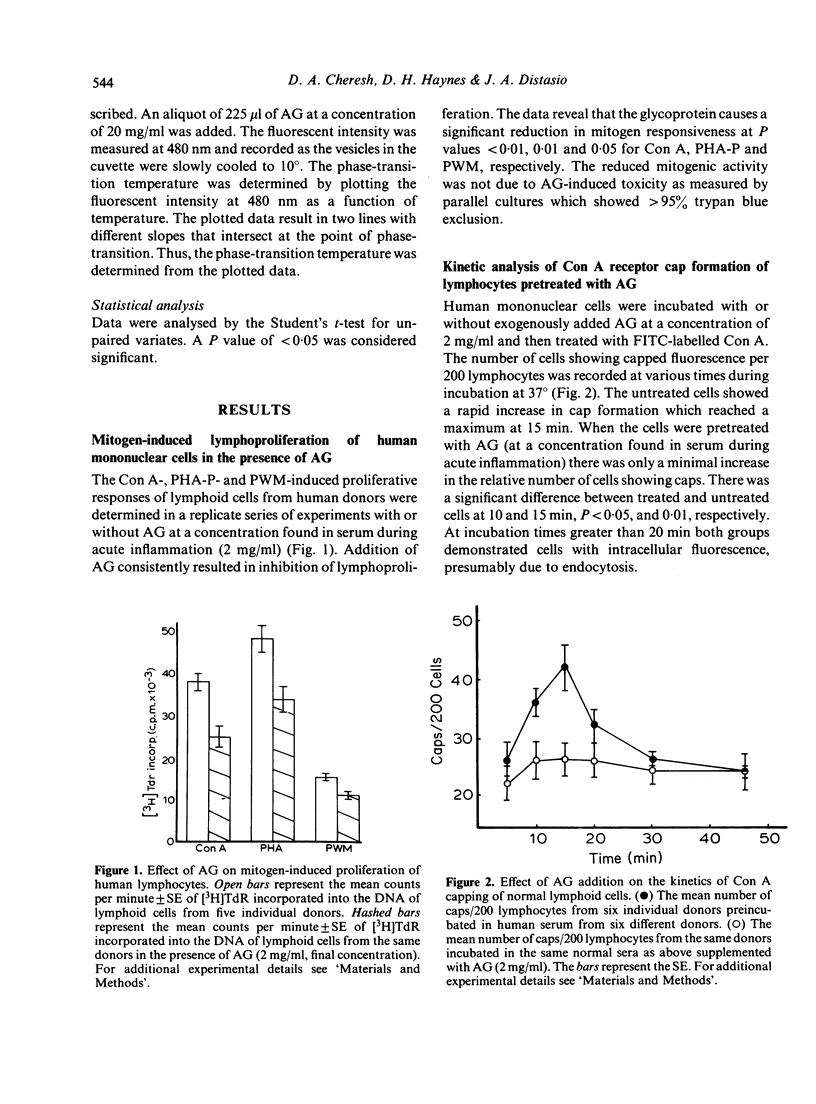
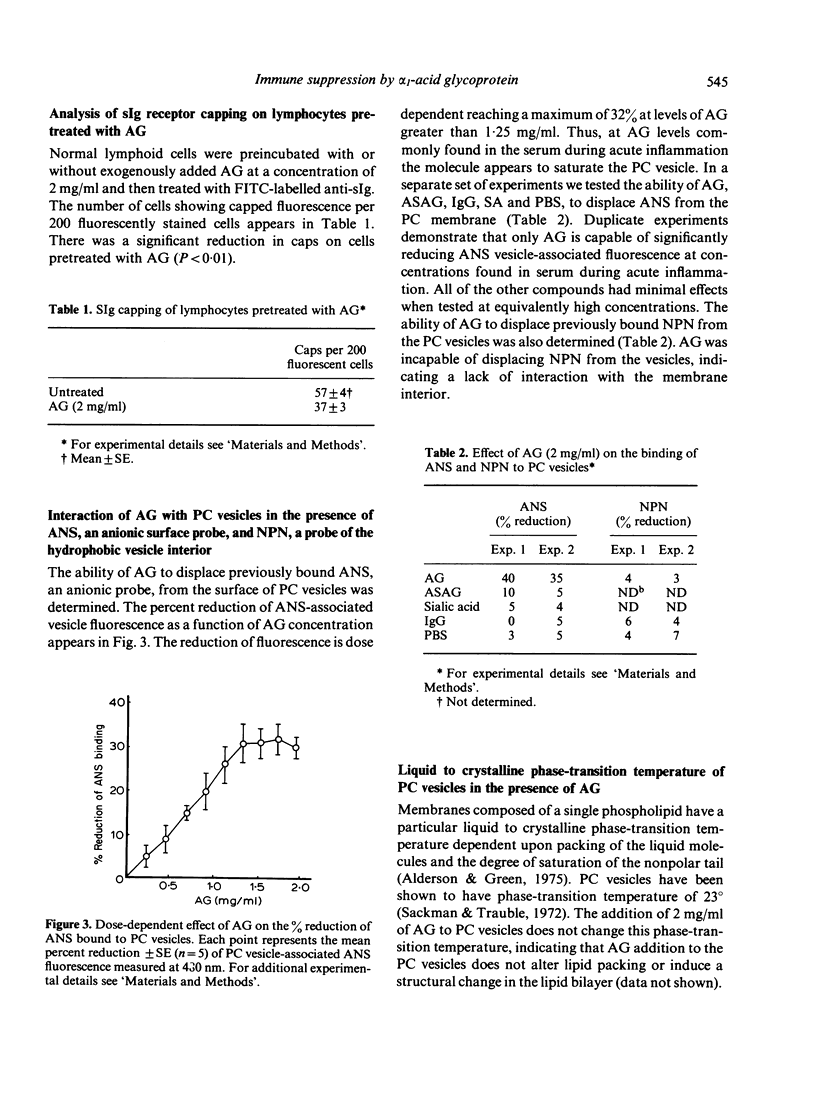
alpha 1-Acid glycoprotein (AG), a serum component elevated during acute inflammation, has been implicated in the suppression of various immunological responses. Pretreatment of lymphoid cells with AG at a concentration commonly found in patients with acute inflammation results in the inhibition of mitogen induced lymphoproliferation as well as capping of concanavalin A (Con A) receptors and surface immunoglobulin (sIg) on the lymphoid cell surface. In order to determine a potential interaction of AG with the lipid bilayer we examined the effects of purified AG on synthetic phosphatidyl choline vesicles. AG displaces 1-anilino-8-naphthalene sulphonate (ANS), an anionic surface probe from these vesicles yet is unable to perturb the binding of N-phenyl-1-naphthalamine (NPN), a hydrophobic probe of the membrane interior. The non-immunosuppressive asialo-derivative of AG is incapable of displacing ANS from the vesicles. The interaction of AG with the membrane may partially involve electrostatic forces mediated by sialic acid and/or steric hindrance of receptor mobility. The results suggest that AG has the capacity to perturb the lymphoid cell surface and interfere with events required for lymphocyte proliferation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson J. C., Green C. Enrichment of lymphocytes with cholesterol and its effect on lymphocyte activation. FEBS Lett. 1975 Apr 1;52(2):208–211. doi: 10.1016/0014-5793(75)80807-6. [DOI] [PubMed] [Google Scholar]
- Bennett M., Schmid K. Immunosuppression by human plasma alpha 1-acid glycoprotein: importance of the carbohydrate moiety. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6109–6113. doi: 10.1073/pnas.77.10.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley W. P., Blasco A. P., Weiss J. F., Alexander J. C., Jr, Silverman N. A., Chretien P. B. Correlations among serum protein-bound carbohydrates, serum glycoproteins, lymphocyte reactivity, and tumors burden in cancer patients. Cancer. 1977 Nov;40(5):2264–2272. doi: 10.1002/1097-0142(197711)40:5<2264::aid-cncr2820400537>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Distasio J. A., Vogel C. L., Lopez D. M. Mitogen-induced blastogenesis and receptor mobility inhibition by breast cancer serum with elevated orosomucoid (alpha 1-acid glycoprotein) levels. J Natl Cancer Inst. 1982 May;68(5):779–783. [PubMed] [Google Scholar]
- Chiu K. M., Mortensen R. F., Osmand A. P., Gewurz H. Interactions of alpha1-acid glycoprotein with the immune system. I. Purification and effects upon lymphocyte responsiveness. Immunology. 1977 Jun;32(6):997–1005. [PMC free article] [PubMed] [Google Scholar]
- Cullen B. F., Chretien P. B., Leventhal B. G. The effect of lignocaine on PHA-stimulated human lymphocyte transformation. Br J Anaesth. 1972 Dec;44(12):1247–1252. doi: 10.1093/bja/44.12.1247. [DOI] [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Flanagan M. T., Hesketh T. R. Electrostatic interactions in the binding of fluorescent probes to lipid membranes. Biochim Biophys Acta. 1973 Mar 29;298(3):535–545. doi: 10.1016/0005-2736(73)90072-2. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Leukocyte surface origin of human alpha1-acid glycoprotein (orosomucoid). J Exp Med. 1978 Aug 1;148(2):507–521. doi: 10.1084/jem.148.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S. C., Woda B. A., Feldman J. D. T lymphocytes of young and aged rats. I. Distribution, density, and capping of T antigens. J Immunol. 1981 Jul;127(1):149–153. [PubMed] [Google Scholar]
- Hallgren H. M., Buckley C. E., 3rd, Gilbertsen V. A., Yunis E. J. Lymphocyte phytohemagglutinin responsiveness, immunoglobulins and autoantibodies in aging humans. J Immunol. 1973 Oct;111(4):1101–1107. [PubMed] [Google Scholar]
- Han T. Enhancement of in vitro lymphocyte response by neuraminidase. Clin Exp Immunol. 1973 Jan;13(1):165–170. [PMC free article] [PubMed] [Google Scholar]
- Haynes D. H. 1-Anilino-8-naphthalenesulfonate: a fluorescent indicator of ion binding electrostatic potential on the membrane surface. J Membr Biol. 1974 Jul 12;17(3):341–366. doi: 10.1007/BF01870191. [DOI] [PubMed] [Google Scholar]
- Haynes D. H., Staerk H. 1-Anilino-8-naphthalenesulfonate: a fluorescent probe of membrane surface structure, composition and mobility. J Membr Biol. 1974 Jul 12;17(3):313–340. doi: 10.1007/BF01870190. [DOI] [PubMed] [Google Scholar]
- Nagura H., Asai J., Katsumata Y., Kojima K. Role of electric surface charge of cell membrane in phagocytosis. Acta Pathol Jpn. 1973 May;23(2):279–290. doi: 10.1111/j.1440-1827.1973.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Noronha A. B., Antel J. P., Roos R. P., Arnason B. G. Changes in concanavalin A capping of human lymphocytes with age. Mech Ageing Dev. 1980 Apr;12(4):331–337. doi: 10.1016/0047-6374(80)90066-4. [DOI] [PubMed] [Google Scholar]
- Sackmann E., Träuble H. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. I. Use of spin labels and optical probes as indicators of the phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4482–4491. doi: 10.1021/ja00768a013. [DOI] [PubMed] [Google Scholar]
- Valet G., Bamberger S., Hofmann H., Schindler R., Ruhenstroth-Bauer G. Flow cytometry as a new method for the measurement of electrophoretic mobility of erythrocytes using membrane charge staining by fluoresceinated polycations. J Histochem Cytochem. 1979 Jan;27(1):342–349. doi: 10.1177/27.1.86564. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Yoshinaga M., Yoshinaga A., Waksman B. H. Regulation of lymphocyte responses in vitro: potentiation and inhibition of rat lymphocyte responses to antigen and mitogens by cytochalasin B. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3251–3255. doi: 10.1073/pnas.69.11.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oss C. J., Gillman C. F., Bronson P. M., Border J. R. Phagocytosis-inhibiting properties of human serum alpha-1 acid glycoprotein. Immunol Commun. 1974;3(4):321–328. doi: 10.3109/08820137409061112. [DOI] [PubMed] [Google Scholar]


