Abstract
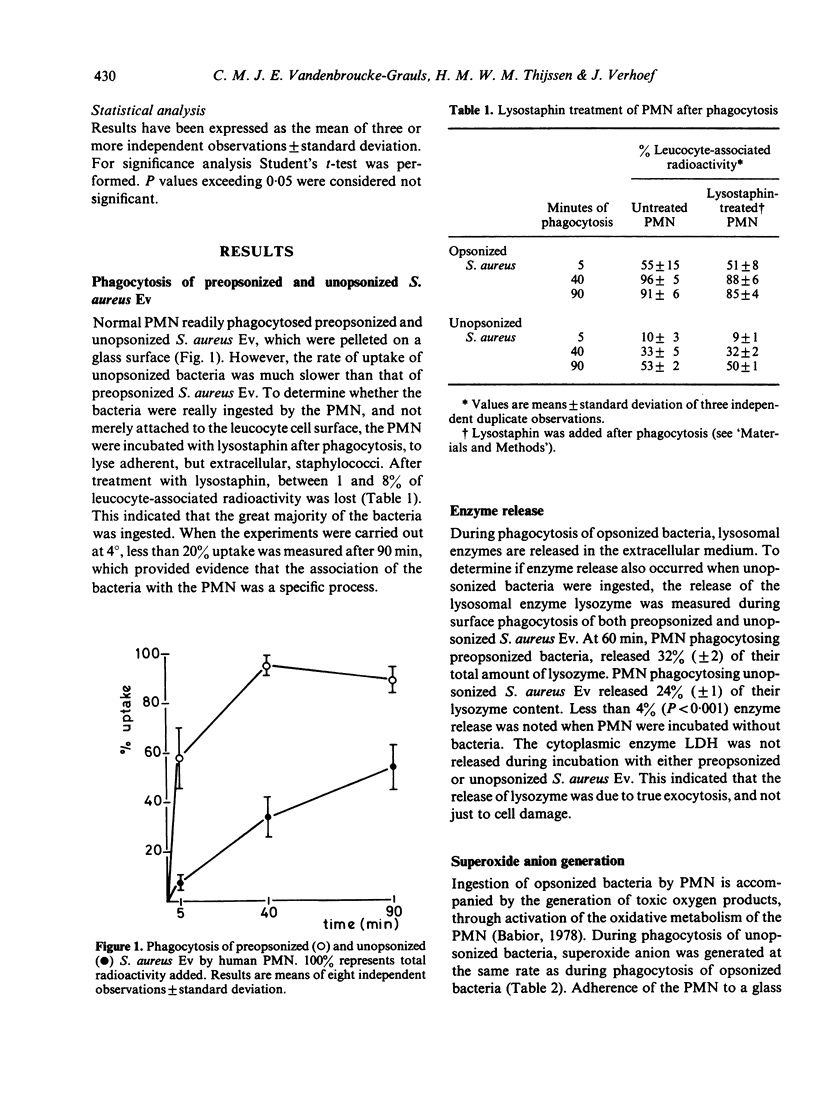
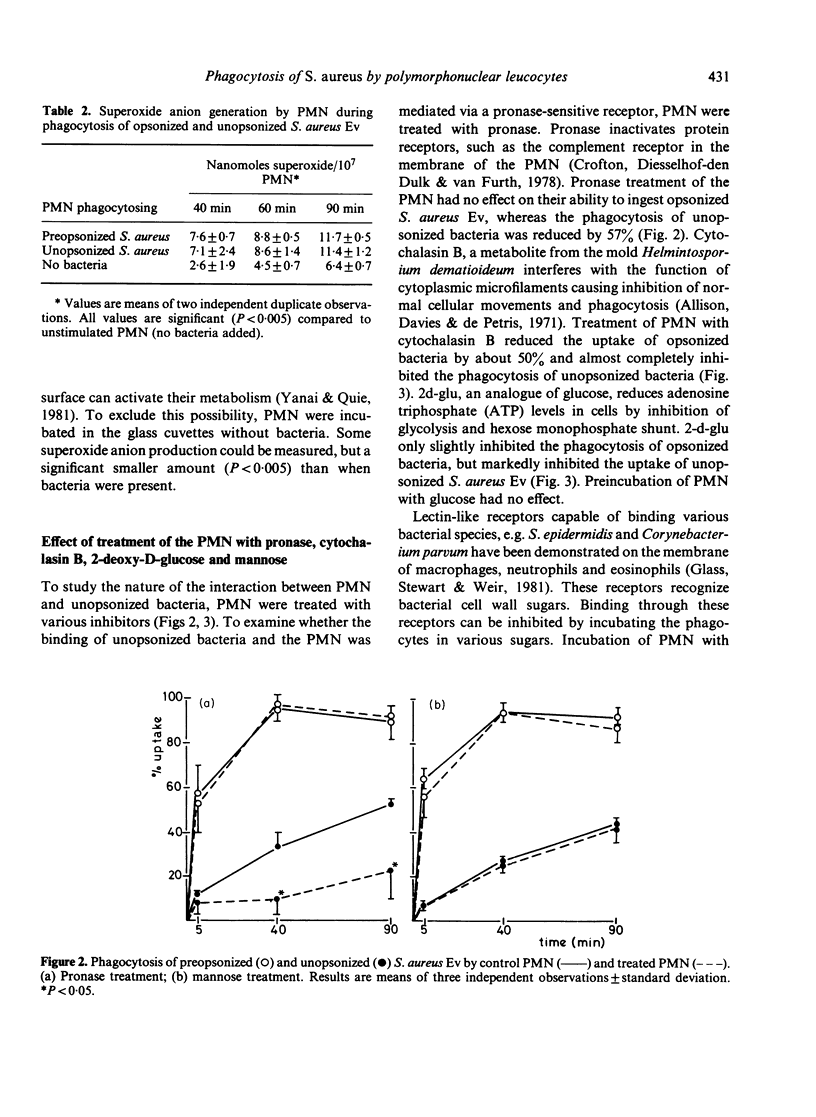
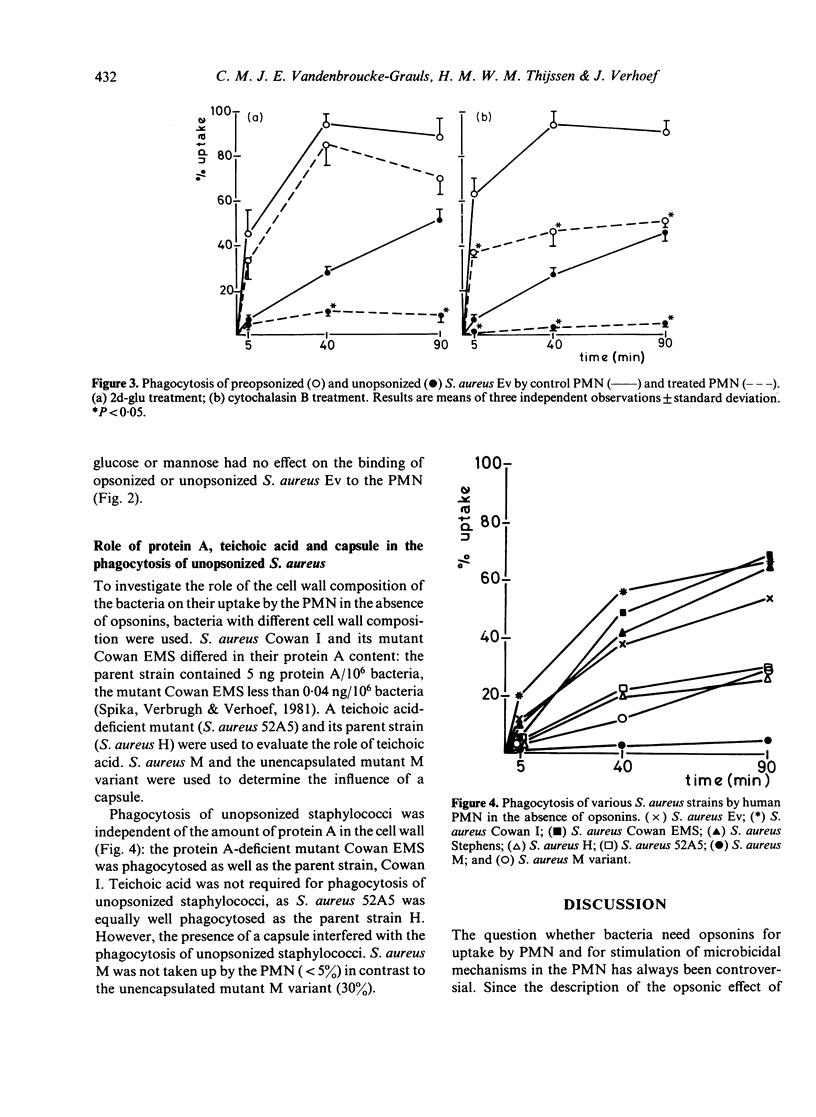
Phagocytosis of Staphylococcus aureus by human polymorphonuclear leucocytes (PMN) in the presence and absence of opsonins was studied with an assay which allows interaction between PMN and bacteria on a surface. The kinetics of uptake, the activity of the metabolic burst, and the degranulation during phagocytosis of opsonized and unopsonized bacteria were compared. Uptake of unopsonized S. aureus proceeded at a slower rate, but unopsonized staphylococci induced metabolic activity and degranulation in the PMN to the same extent as opsonized bacteria. Treatment of PMN with a metabolic inhibitor (2-deoxy-D-glucose) or with an inhibitor of microfilament function (cytochalasin B) totally inhibited the capacity of PMN to ingest unopsonized S. aureus, whereas uptake of opsonized bacteria was much less affected. Treatment of the PMN with pronase prevented uptake of unopsonized bacteria, but had no effect on the uptake of opsonized bacteria. Uptake was not inhibited by mannose. Recognition of S. aureus by the PMN was not dependent on the presence of the cell wall components protein A or teichoic acid. The presence of a capsule inhibited uptake.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., De Petris S. Role of contractile microfilaments in macrophage movement and endocytosis. Nat New Biol. 1971 Aug 4;232(31):153–155. doi: 10.1038/newbio232153a0. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Daimond R. D., Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro. J Clin Invest. 1978 Feb;61(2):360–369. doi: 10.1172/JCI108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass E., Stewart J., Weir D. M. Presence of bacterial binding 'lectin-like' receptors on phagocytes. Immunology. 1981 Nov;44(3):529–534. [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudewicz P. W., Beezhold D. H., Van Alten P., Molnar J. Lack of stimulation of post-phagocytic metabolic activities of polymorphonuclear leukocytes by fibronectin opsonized particles. J Reticuloendothel Soc. 1982 Aug;32(2):143–154. [PubMed] [Google Scholar]
- HIRSCH J. G., COHN Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med. 1960 Dec 1;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hed J., Stendahl O. Differences in the ingestion mechanisms of IgG and C3b particles in phagocytosis by neutrophils. Immunology. 1982 Apr;45(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Henricks P. A., van Erne-van der Tol M. E., Verhoef J. Partial removal of sialic acid enhances phagocytosis and the generation of superoxide and chemiluminescence by polymorphonuclear leukocytes. J Immunol. 1982 Aug;129(2):745–750. [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of microorganisms. Rev Infect Dis. 1982 Jan-Feb;4(1):104–123. doi: 10.1093/clinids/4.1.104. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. A., Hoidal J. R., Clawson C. C., Quie P. G., Peterson P. K. Phagocytosis by polymorphonuclear leukocytes of Staphylococcus aureus and Pseudomonas aeruginosa adherent to plastic, agar, or glass. J Immunol Methods. 1983 Sep 30;63(1):103–114. doi: 10.1016/0022-1759(83)90213-2. [DOI] [PubMed] [Google Scholar]
- Ogmundsdóttir H. M., Weir D. M. The characteristics of binding of Corynebacterium parvum to glass-adherent mouse peritoneal exudate cells. Clin Exp Immunol. 1976 Nov;26(2):334–339. [PMC free article] [PubMed] [Google Scholar]
- Ohman L., Hed J., Stendahl O. Interaction between human polymorphonuclear leukocytes and two different strains of type 1 fimbriae-bearing Escherichia coli. J Infect Dis. 1982 Dec;146(6):751–757. doi: 10.1093/infdis/146.6.751. [DOI] [PubMed] [Google Scholar]
- Spika J. S., Verbrugh H. A., Verhoef J. Protein A effect on alternative pathway complement activation and opsonization of Staphylococcus aureus. Infect Immun. 1981 Nov;34(2):455–460. doi: 10.1128/iai.34.2.455-460.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Edebo L. Phagocytosis of mutants of Salmonella typhimurium by rabbit polymorphonuclear cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):481–488. doi: 10.1111/j.1699-0463.1972.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. II. Contact angles and phagocytosis of encapsulated bacteria before and after opsonization by specific antiserum and complement. J Reticuloendothel Soc. 1972 Nov;12(5):497–502. [PubMed] [Google Scholar]
- Verbrugh H. A., Hoidal J. R., Nguyen B. Y., Verhoef J., Quie P. G., Peterson P. K. Human alveolar macrophage cytophilic immunoglobulin G-mediated phagocytosis of protein A-positive staphylococci. J Clin Invest. 1982 Jan;69(1):63–74. doi: 10.1172/JCI110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Peters R., Peterson P. K., Verhoef J. Phagocytosis and killing of staphylococci by human polymorphonuclear and mononuclear leucocytes. J Clin Pathol. 1978 Jun;31(6):539–545. doi: 10.1136/jcp.31.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Van Dijk W. C., Peters R., Van Der Tol M. E., Peterson P. K., Verhoef J. Staphylococcus aureus opsonization mediated via the classical and alternative complement pathways. A kinetic study using MgEGTA chelated serum and human sera deficient in IgG and complement factors C1s and C2. Immunology. 1979 Mar;36(3):391–397. [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P., Kim Y., Sabath L. D., Quie P. G. Opsonic requirements for staphylococcal phagocytosis. Heterogeneity among strains. Immunology. 1977 Aug;33(2):191–197. [PMC free article] [PubMed] [Google Scholar]
- Yanai M., Quie P. G. Chemiluminescence by polymorphonuclear leukocytes adhering to surfaces. Infect Immun. 1981 Jun;32(3):1181–1186. doi: 10.1128/iai.32.3.1181-1186.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


