Abstract
Eph tyrosine kinase receptors and their membrane-bound ligands, ephrins, are presumed to regulate cell–cell interactions. The major consequence of bidirectional activation of Eph receptors and ephrin ligands is cell repulsion. In this study, we discovered that Xenopus Dishevelled (Xdsh) forms a complex with Eph receptors and ephrin-B ligands and mediates the cell repulsion induced by Eph and ephrin. In vitro re-aggregation assays with Xenopus animal cap explants revealed that co-expression of a dominant-negative mutant of Xdsh affected the sorting of cells expressing EphB2 and those expressing ephrin-B1. Co-expression of Xdsh induced the activation of RhoA and Rho kinase in the EphB2-overexpressed cells and in the cells expressing EphB2-stimulated ephrin-B1. Therefore, Xdsh mediates both forward and reverse signaling of EphB2 and ephrin-B1, leading to the activation of RhoA and its effector protein Rho kinase. The inhibition of RhoA activity in animal caps significantly prevents the EphB2- and ephrin-B1-mediated cell sorting. We propose that Xdsh, which is expressed in various tissues, is involved in EphB and ephrin-B signaling related to regulation of cell repulsion via modification of RhoA activity.
Keywords: Dishevelled/Eph/ephrin/hindbrain/repulsion
Introduction
The members of the Eph receptors family can be classified into two groups based on their sequence similarity and their preferential binding to a subset of ligands tethered to the cell surface by either a glycosylphosphatidyl inositol anchor (ephrin-A) or a transmembrane domain (ephrin-B). EphB2 associates with the transmembrane (TM) ligands ephrin-B1, ephrin-B2 and ephrin-B3, while ephrin-B1 associates with EphB1, EphB2 and EphB3 (Orioli and Klein, 1997; Pasquale, 1997). Ephrin-B ligands have been shown to be phosphorylated on tyrosine residues after contact with the corresponding Eph receptor ectodomain and to have a receptor-like intrinsic signaling potential leading to transduction of reverse signaling (Holland et al., 1996; Bruckner et al., 1997).
The major consequence of this bidirectional activation of Eph receptors and ephrin ligands is cell repulsion. For example, the repulsion of axons expressing Eph receptors from ephrin-expressing regions is required for the correct axon pathfinding in the commisure axons (Henkemeyer et al., 1996; Orioli et al., 1996; Kullander et al., 2001) and the retino-tectal projection of retinal ganglion cells (Goodhill and Richards, 1999). Another striking example is the restriction of cell intermingling between adjacent segments in vertebrate hindbrain. The developing hindbrain is subdivided transiently into structural repeat units, rhombomeres whose borders are strictly regulated. During normal Xenopus development, Eph receptors and ephrin-B ligands are expressed in alternate segments of the hindbrain by the early tailbud stage, and this alternate expression of Eph and ephrin is important in maintaining a clear boundary between adjacent rhombomeres (Xu et al., 1995, 2000).
Repulsive cell movement is generated by the local modulation of cell dynamics that promotes cells growing away from the guiding stimuli. However, the molecular modules that deliver positional guidance cues from the cell surface receptors and ligands to the actin cytoskeleton have not been clearly identified. It is important to identify the intracellular targets of activated Eph receptors and ephrins and elucidate how they are committed to repulsive cell movements. In this report, we show that Xenopus Dishevelled (Xdsh) forms a complex with EphB receptors and also with ephrin-B1. Xdsh is involved in Eph- and ephrin-mediated cell repulsion as a downstream effector of these receptors and ligand. At present, Rho family GTPases are the major molecules which are reported to mediate the repulsive cell movement induced by Eph and ephrin. Activation of the EphA receptors of retinal ganglion cells by the ephrin-A5–Fc fusion protein has been found to induce activation of RhoA and Rho kinase, leading to growth cone collapse (Wahl et al., 2000). Moreover, Shamah et al. (2001) recently cloned ephexin, a guanine nucleotide exchange factor for RhoA, Rac1 and Cdc42, that directly interacts with EphA receptors. Here, we found that activation of EphB2 receptor or ephrin-B1 ligand leads to the activation of RhoA by co-expression with Xdsh.
Dishevelled (Dsh) is a cytoplasmic protein containing three conserved protein domains: DIX (Dishevelled-Axin), PDZ and DEP. Dsh is known to be located as a downstream molecule of Frizzled (Frz) and involved in at least two independent signaling pathways in Drosophila, Xenopus and zebrafish. One pathway is a canonical cascade that leads to the dorsalization and determination of the body axis by interacting with β-catenin (Sakanaka et al., 2000), and the other is a planar cell polarity (PCP) cascade. In vertebrates, the PCP pathway is involved in the convergent and extension movements of both axial mesoderm and neuroectodermal cells during gastrulation (Heisenberg et al., 2000; Wallingford et al., 2000; Wallingford and Harland, 2001). It has never been reported that Xdsh has any functions as a downstream molecule of receptor tyrosine kinases or their ligands. Our finding that Xdsh is involved in both the forward and reverse signaling induced by Eph and ephrin shows a novel role for Xdsh in signal transduction and development.
In the present study, we used Xenopus embryo and showed that Xdsh associates with EphB1, EphB2 and ephrin-B1. Co-expression of the dominant-negative mutant of Xdsh (Xdd1) affected sorting-out of cells expressing EphB2 and ephrin-B1 from those expressing their corresponding ligands and receptors, respectively. The co-expression of Xdsh with activated EphB2 receptor or activated ephrin-B1 ligand enhanced the activities of RhoA and its effector Rho kinase. The RhoA activation via Xdsh at the restricted region of cell boundaries where contact between EphB and ephrin-B takes place is considered to be necessary for sorting out the receptor- and ligand-expressing cells.
Results
Xdsh forms a complex with EphB receptors and ephrin-B ligands
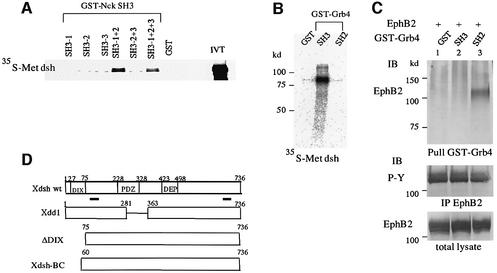
Since EphB receptors and ephrin-B ligands are known to interact with SH adaptors (Stein et al., 1998; Cowan and Henkemeyer, 2001), we first attempted to determine whether Xdsh could be a downstream molecule of Eph and ephrin by interacting with the SH adaptors. As shown in Figure 1A, when Xdsh was transcribed and translated in vitro, the translation product bound well to the GST fusion containing the first and the second SH3 domains of Nck (Nck SH3-1 + 2). Direct association of Xdsh with the SH3 domains of Grb4 but not the SH2 domain was also detected by the same analysis (Figure 1B). These results indicate that the SH3 domains of Nck and Grb4 bind directly to Xdsh via the proline-rich regions within Xdsh (Figure 1D). Furthermore, in addition to the previous discovery that Grb4 interacts with ephrin-B ligands (Cowan and Henkemeyer, 2001), we demonstrated that the SH2 domain of Grb4 binds to the tyrosine-phosphorylated EphB2 receptor. EphB2 is autophosphorylated at tyrosine residues when it is overexpressed in 293T cells (Figure 1C). The GST-tagged SH2 domain of Grb4 pulled EphB2 from cell lysate in which EphB2 was autophosphorylated (Figure 1C).
Fig. 1. Xdsh binds to Nck and Grb4 in vitro. (A) Full-length Xenopus cDNA encoding Dsh was subjected to in vitro transcription and translation, and [35S]methionine-labeled translation products were incubated with glutathione–agarose beads bound to GST–Nck SH3-1, GST–Nck SH3-2, GST–Nck SH3-3, GST–Nck SH3-1 + 2, GST–Nck SH3-2 + 3, GST–Nck SH3-1 + 2 + 3 or to GST as indicated above the lanes. (B) [35S]methionine-labeled Xdsh was incubated with GST, GST–Grb4 SH3 domains or the GST–Grb4 SH2 domain, as marked. Beads were washed, and bound proteins were separated by SDS–PAGE and detected by autoradiography. IVT, input in vitro translation reaction before bead binding. (C) Total cell lysates from EphB2-overexpressing 293T cells were incubated with glutathione–agarose-conjugated GST, GST–Grb4 SH3 domains and the GST–Grb4 SH2 domain, as indicated. Beads were washed, and the co-precipitated EphB2 protein was detected by immunoblot. The expression of EphB2 and the phosphorylation of EphB2 are shown at the bottom by anti-EphB2 and anti-phosphotyrosine antibodies. (D) Schematic representation of the wild-type and truncated Xdsh cDNA constructs used in this study. Proteins are depicted to scale; underlining indicates proline-rich regions.
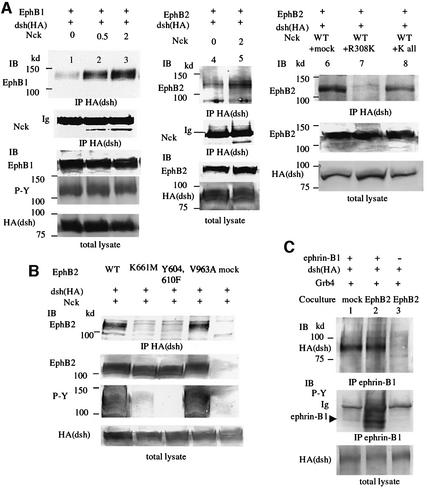
Co-precipitation analysis revealed that Xdsh formed a complex with the autophosphorylated EphB1 or EphB2 receptor when the Nck expression level was increased (Figure 2A, lanes 1–5). Co-expression of an excess volume of Nck carrying a mutation in the SH2 domain that impaired its binding to the EphB receptors (R308K) inhibited the association of Xdsh with the EphB2 receptor (Figure 2A, lane 7), implying that the interaction between Xdsh and EphB receptors requires the presence of SH adaptors. Another Nck mutant, in which all SH3 and SH2 domains are mutated, NckKall, was used as a negative control for non-specific effects of exogenous protein expression (Figure 2A, lane 8). Use of the wild type and various mutants of EphB2 showed that kinase-inactivated EphB2 (K661M) or EphB2 with mutations in tyrosine residues of the cytoplasmic juxtamembrane region (Y604,610F) does not associate with Xdsh, while EphB2 with a mutation in its PDZ domain-binding motif at the C-terminus (V963A) does (Figure 2B). These results may indicate that the association of Xdsh with EphB2 depends on the tyrosine phosphorylation of EphB2, but the PDZ domain of Xdsh is not involved in the association. On the other hand, Xdsh forms a complex with ephrin-B1 without requiring phosphorylation of ephrin-B1. When ephrin-B1-expressing cells were co-cultured with cells expressing EphB2, ephrin-B1 was highly phosphorylated on tyrosine residues, but not when co-cultured with control mock-transfected cells (Figure 2C, lanes 1 and 2). Xdsh was co-precipitated with tyrosine-phosphorylated ephrin-B1 as well as with non-phosphorylated ephrin-B1 (Figure 2C, lanes 1 and 2). In the experiments shown in Figure 2C, we used 293T cells stably expressing EphB2 K661M to stimulate ephrin-B1. Because Xdsh does not bind to EphB2 K661M (Figure 2B) we could exclude the possibility that Xdsh, which co-immunoprecipitated with ephrin-B1 in Figure 2C, was derived from the complex with Eph receptor co-precipitated with the ligand. Similarly, Xdsh also forms a complex with ephrin-B2 in vivo, regardless of phosphorylation of the tyrosine residues of ephrin-B2 (data not shown). These results suggest the constitive association of Xdsh and ephrin-B1.
Fig. 2. Xdsh forms a complex with EphB receptors and ephrin-B1. (A) 293T cells were transiently transfected with plasmid encoding HA-tagged Xdsh together with Nck and EphB1 (lanes 1–3) or EphB2 (lanes 4–8) as indicated above the lanes. In lanes 2, 3 and 5, 0.5 or 2 µg of Nck was co-transfected as indicated. In lanes 7 and 8, 1 µg of wild-type Nck and an excess volume (4 µg) of mutated Nck constructs were co-transfected, as indicated. Nck R308K has a mutation in its SH2 domain, and K all indicates functionally inactivated Nck with a mutation in all SH3 domains and in the SH2 domain. Cells were lysed and immunoprecipitated (IP) with anti-HA, which reacts with the epitopes located at the N-terminus of Xdsh. Bound proteins were immunoblotted (IB) with the antibodies indicated. Ig, immunoglobulin. Expressions of Xdsh and EphB1 or EphB2 in individual cell lysates (total lysate) was confirmed by immunoblotting (bottom). (B) 293T cells were transiently transfected with HA-tagged Xdsh together with Nck and wild-type or various mutants of EphB2, as indicated above the lanes. Xdsh was immunoprecipitated with anti-HA, and co-precipitated EphB2 was detected by immunoblot. The tyrosine phosphorylation (P-Y) of each EphB2 construct is shown at the bottom by anti-phosphotyrosine antibody (4G10). (C) 293T cells stably expressing ephrin-B1 (lanes 1 and 2) or parent 293T cells (lane 3) were transiently transfected with plasmids encoding HA-tagged Xdsh together with Grb4. Transfected cells were co-cultured with mock-transfected 293T cells (lane 1) or 293T cells stably expressing EphB2 K661M (lanes 2 and 3) for 30 min before preparing cell lysates for immunoprecipitation with anti-ephrin-B1. Co-precipitated Xdsh was immunoblotted with anti-HA. The phosphorylation of ephrin-B1 was shown as P-Y by anti-phosphotyrosine antibody (4G10). Ig, immunoglobulin.
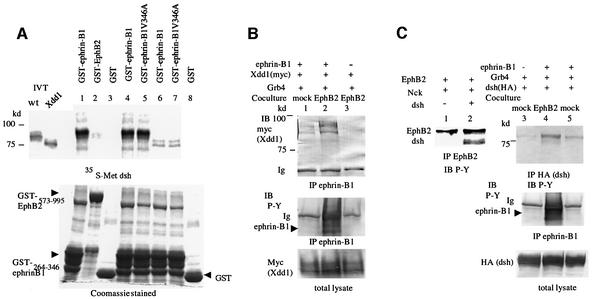
The direct interaction between Xdsh and the cytoplasmic region of ephrin-B1 was confirmed by an in vitro binding assay, as shown in Figure 3A. In vitro translated Xdsh was co-precipitated with GST-tagged ephrin-B1, but not by the control GST alone (Figure 3A, lanes 1 and 3). On the other hand, the cytoplasmic region of EphB2 apparently did not bind to Xdsh directly (Figure 3A, lane 2). Ephrin-B1 with a mutation in its PDZ domain-binding motif also binds to Xdsh the same as wild-type ephrin-B1 (Figure 3A, compare lanes 4 and 5). Therefore, the interaction of Xdsh and ephrin-B1 was direct, but the PDZ domain of Xdsh was not involved in this association. Xdd1 (Figure 1D) is a mutant of Xdsh that is well known to inhibit both the canonical cascade and the PCP cascade (Sokol, 1996; Wallingford et al., 2000), and it is widely used as a dominant-negative form of Xdsh. Although Xdd1 did not bind significantly to ephrin-B1 in vitro compared with wild-type Xdsh (Figure 3A, compare lanes 6 and 7, and lanes 4 and 5), an in vivo co-precipitation assay revealed that Xdd1 forms a complex with ephrin-B1 whose cytoplasmic tyrosine residues are phosphorylated by stimulation of EphB2 (Figure 3B, lane 2). The increased expression level of Grb4 enhanced the association of Xdd1 with tyrosine-phosphorylated ephrin-B1 (data not shown).
Fig. 3. (A) [35S]methionine-labeled full-length (wt, lanes 1, 2, 3, 4 and 5) or Xdd1 mutant Xdsh (lanes 6, 7 and 8) were incubated with glutathione– agarose-conjugated GST (lanes 3 and 8), GST–ephrin-B1264–346 (lanes 1, 4 and 6), GST–ephrin-B1264–346 V346A (lanes 5 and 7) or GST–EphB2573–995 (lane 2). After washing the beads, bound proteins were separated by SDS–PAGE and detected by autoradiography. IVT, input in vitro translation reaction before bead binding. Expression of GST fusion proteins is shown in the Coomassie Blue-stained gel at the bottom. (B) 293T cells stably expressing ephrin-B1 (lanes 1 and 2) or parent 293T cells (lane 3) were transiently transfected with myc-tagged Xdd1 together with Grb4. Transfected cells were co-cultured with mock-transfected 293T cells (lane 1) or 293T cells stably expressing EphB2 K661M (lanes 2 and 3) for 30 min before preparing cell lysates for immunoprecipitation with anti-ephrin-B1. Co-precipitated Xdd1 was immunoblotted with anti-myc. The phosphorylation of ephrin-B1 was shown as P-Y by anti-phosphotyrosine antibody (4G10). Ig, immunoglobulin. (C) 293T cells were transiently transfected with EphB2 and Nck with (lanes 1 and 2) or without Xdsh (lanes 3–5). 293T cells stably expressing ephrin-B1 (lanes 4 and 5) or parent 293T cells (lane 3) were transiently transfected with HA-tagged Xdsh and Grb4. Transfected cells were co-cultured with mock-tranfected 293T cells (lanes 3 and 5) or 293T cells stably expressing EphB2 K661M (lane 4) as in (B). Cell lysates were immunoprecipitated with anti-EphB2 (lanes 1 and 2) or anti-HA (lanes 3–5), and precipitated proteins were immunoblotted with anti-phosphotyrosine (P-Y: 4G10).
Taken together, these results indicate that Xdsh interacts with tyrosine-phosphorylated EphB1 and EphB2 via association with the SH adaptor Nck or Grb4, although the possibility of direct interaction between Xdsh and EphB receptors has not been completely eliminated. There are two possible mechanisms for recruitment of Xdsh to ephrin-B1: a constitutive direct association, and that through an interaction with Grb4 when ephrin-B1 is phosphorylated by EphB receptors. Next, we examined the phosphorylation of Xdsh when it interacts with EphB2 or ephrin-B1. Xdsh co-precipitated with overexpressed EphB2 was highly phosphorylated at tyrosine residues (Figure 3C, lane 2). Likewise, when ephrin-B1 was not stimulated by EphB receptors, the phosphorylation of Xdsh was very weak, in spite of the constitutive association between Xdsh and ephrin-B1 (Figure 3C, lane 5). However, the tyrosine residues of Xdsh were phosphorylated when ephrin-B1 was phosphorylated by coming into contact with EphB2-expressing cells (Figure 3C, lane 4). Therefore, as a consequence of the association with EphB2 or EphB2-stimulated ephrin-B1, the tyrosine residues of Xdsh were phosphorylated, although the significance of tyrosine phosphorylation of Xdsh is not fully understood.
A dominant-negative Xdsh mutant affected the cell sorting of EphB- and ephrin B1-expressing cells in vitro
Two methods using Xenopus animal cap cells were used to determine whether Xdsh mediates the restriction of intermingling of EphB- and ephrin-B-expressing cells. One method was a cell aggregation assay in which cells from animal cap explants from embryos microinjected with EphB receptor or ephrin-B1, together with a different colored fluorescence-conjugated dextran, were mixed in equal proportions in vitro and allowed to aggregate for several hours.
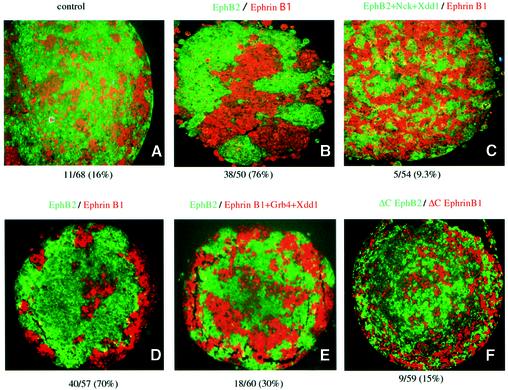
EphB2- and ephrin B1-expressing cells sorted themselves out from each other, creating a distinct segregated pattern of different colored cell masses, in contrast to the mixed colored pattern of cells in a control aggregate injected with only fluorescent dextrans (compare Figure 4A and B with D). The sorting of EphB- and ephrinB1-expressing cells was thought to be attributable to downstream signaling of the receptor and ligand, because cells expressing truncated mutants of EphB2 (ΔC EphB2) and ephrin-B1 (ΔC ephrin-B1), which lack the cytoplasmic region of each protein, did not elicit this restriction (Figure 4F). Co-expression of the dominant-negative mutant of Xdsh (Xdd1) together with EphB2 affected the cell sorting, leading to the formation of a mixed pattern of two sets of colored cells (Figure 4C). Co-expression of Xdd1 with ephrin-B1 inhibited the cell sorting less effectively than it did with EphB2, but the mixing was still evident when compared with the clear segregation pattern observed in the control (compare Figure 4D and E). As reported, endogenous Xdsh is ubiquitous in embryos in the early stages of development, including the animal ectodermal region, which is fated to become the animal cap used in this assay (data not shown) (Sokol et al., 1995). Therefore, our observation described above indicates that Xdd1 blocked the endogenous Xdsh-mediated cell sorting induced by Eph and ephrin.
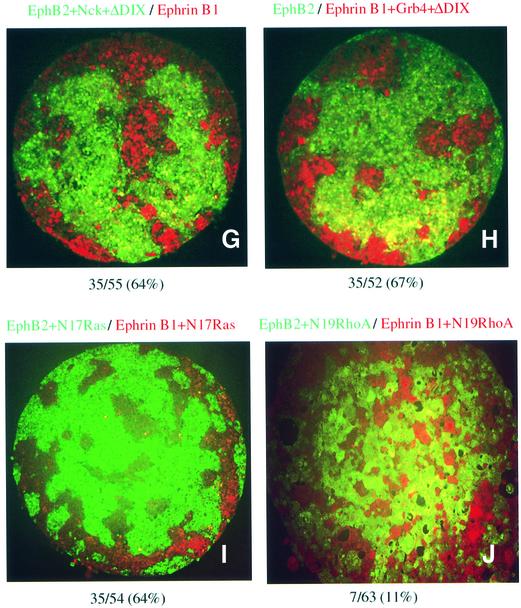
Fig. 4. Xdd1 affects EphB2- and ephrin-B1-induced cell sorting by in vitro aggregation assay. Cells from animal cap explants from embryos microinjected with RNA as indicated at the top together with either Oregon green or Texas red tracer fluorescence as described in Materials and methods were dissociated, mixed in equal proportions in vitro, and allowed to re-aggregate for 8 h. The re-aggregates were sectioned, and the pattern of the cells was examined histologically. Similar results were obtained in at least three independent experiments, and typical results are shown. The results in (A–C), (D–F) and (G–J) were obtained from separate experiments. (A) Control; embryos were microinjected with tracer fluorescence only. The composition of the aggregates was assessed as described in Materials and methods as either segregated or mixed. The ratio (percentage) of the segregated units to the total area is shown at the bottom.
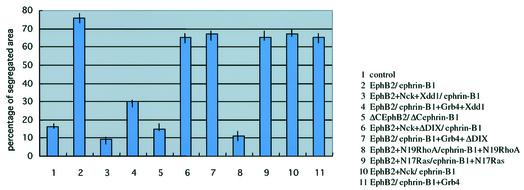
Next, we examined the effect of another mutant of Xdsh, ΔDIX, which lacks the DIX domain of Xdsh, and selectively blocks the canonical signaling, but not the PCP cascade. As shown in Figure 4G and H, co-expression of ΔDIX with EphB2 or ephrin-B1 did not affect cell sorting. This result suggests that canonical signaling of Xdsh is not involved in Eph- and ephrin-mediated cell repulsion. This conclusion was also confirmed by using Xdsh-BC, which lacks almost all of the DIX domain, and thus blocks the canonical cascade (Itoh et al., 2000). The co-injection of Xdsh-BC with EphB2 or ephrin-B1 also did not affect the cell sorting (data not shown). Finally, we examined whether blocking of the signaling cascade of RhoA or Ras affects the cell repulsion induced by Eph and ephrin. Co-expression of a dominant-negative mutant of RhoA (N19RhoA) significantly affected the repulsive cell movement, whereas dominant-negative Ras did not (Figure 4I and J). These results are summarized in Figure 5 with quantitation of the segregated area in each aggregate as described in Materials and methods. Because we have co-injected Nck or Grb4 together with Xdd1 to connect Xdd1 to EphB2 or ephrin-B1, we tested whether Nck alone affects EphB2-mediated repulsion and whether Grb4 alone affects ephrin-B1-mediated repulsion. We found that Nck alone and Grb4 alone (without Xdsh) did not affect the cell sorting (Figure 5, columns 10 and 11). We reasoned that these SH adaptors do not block binding sites that connect EphB2 and ephrin-B1 to critical downstream effctors other than Xdsh.
Fig. 5. Quantitative analysis of the segregation ratio in animal cap aggregation assay. Each column represents the area with a segregated pattern as a percentage of the total area of animal cap aggregates. The quantitation was performed as described in Materials and methods. The bar indicates the range of the percentage in at least three independent experiments.
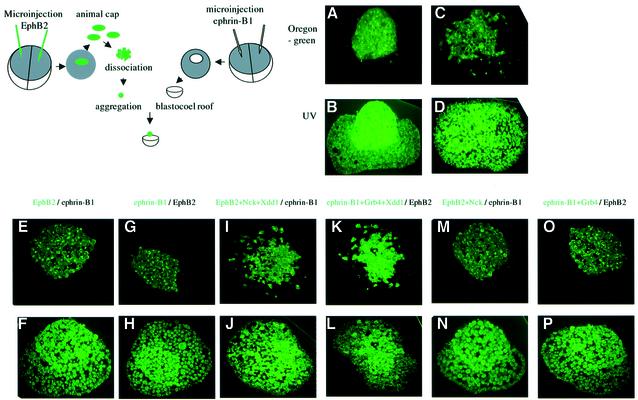
For the next demonstration, we determined whether Xdd1 influences the aggregation of inner cells of animal explants expressing EphB2 or ephrin-B1 sorted out from the blastocoel roof (BCR) layer, in which the corresponding ligand or receptor was introduced exogenously (see the scheme in Figure 6; and as described in Materials and methods). The cell aggregates from the ectodermal explants sank into the explanted BCR during incubation, as described in a recent report (Winklbauer et al., 2001). Although the inner cells of the animal explants injected with fluorescent lineage tracer only initially formed a clear border from the explanted BCR derived from an uninjected control embryo (Figure 6A), they intermingled after incubation for 3.5 h (Figure 6C). In contrast, a distinct border persisted even after an incubation period of ≥3.5 h in the BCR layers in which ephrin-B1 and EphB2, respectively, were expressed (Figure 6E and G). This restriction of the border was affected by co-expression of Xdd1 together with EphB2 or ephrin-B1, as shown in Figure 6I and K. Moreover, Nck alone did not affect the EphB2-expressed aggregate being sorted out from BCR-expressing ephrin-B1. Similarly, Grb4 alone did not affect the ephrin-B1-expressed aggregate being sorted out from EphB2-expressing BCR (Figure 6M and O). These results further confirmed that Xdsh mediates EphB2- and ephrin-B1-mediated repulsive cell movement. These findings also verified that the inhibition of cell sorting between EphB2- and ephrin B1-expressing cells was not attributable to the generalized inhibitory effect of Xdd1 on cell motility. It is because the effect we observed in Figure 6 was the specific result of the migration of Xdd1-injected cells beyond the border and crossing over into the BCR.
Fig. 6. Co-expression of Xdd1 affects cell repulsion by in vitro blastocoel roof assay. Animal cap explants from embryos microinjected with Oregon green-conjugated dextran (A–D) or together with RNA encoding EphB2 (E and F), ephrin-B1 (G and H), EphB2 plus Nck and Xdd1 (I and J), ephrin-B1 plus Grb4 and Xdd1 (K and L), EphB2 plus Nck (M and N) or ephrin-B1 plus Grb4 (O and P) were dissected and dissociated at stage 9. Inner cells were then kept aggregated for 30 min and stayed on the surface of the blastocoel roof derived from embryos microinjected with ephrin-B1 (E, F, I, J, M and N), EphB2 (G, H, K, L, O and P) or control uninjected embryos (A, B, C and D), and cultured for 15 min (A and B) or 3.5 h (C–P). Sections of each sample were examined histologically with Oregon green (A, C, E, G, I, K, M and O) or with UV radiation to determine its entire morphology (B, D, F, H, J, L, N and P). Similar results were obtained in at least 10 independent BCR assays. The illustrated scheme shows the strategy of the experiment as a sample case shown (E) and (F).
Xdsh mediates RhoA and Rho kinase activity by interaction with EphB receptors and ephrin-B ligands
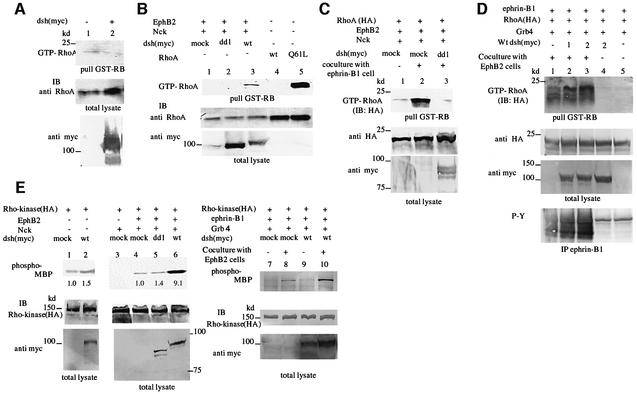
Next, we examined how Xdsh modifies cell sorting between EphB- and ephrin-B-expressing cells. Although the molecules responsible for the repulsion between their receptors and ligands have not been clearly identified, Rho family GTPases, known to be involved in various cell movement dynamics, are reasonable candidates in this context. Since RhoA and its effector protein Rho kinase are known to be activated by Wnt/Frz signaling via Dsh (Fanto et al., 2000; Strutt, 2001; Winter et al., 2001), we next investigated whether Xdsh modifies the activity of RhoA and Rho kinase by interaction with Eph and ephrin. The activation of RhoA in vivo was examined by affinity precipitation of GTP-bound RhoA with the GST fusion protein of the RhoA-binding domain of Rho kinase (GST–RB) (Matsui et al., 1996). Activated RhoA was not detected in 293T cells transfected with Xdsh alone (Figure 7A, compare lanes 1 and 2) or EphB2 alone (Figure 7B, lane 1). However, RhoA was clearly activated by co-expression of EphB2 together with wild-type Xdsh (Figure 7B, lane 3). Although the overexpression of EphB2 alone did not increase the GTP-bound RhoA, the ligand stimulation of EphB2 by co-culture with 293T cells stably expressing ephrin-B1 leads to activation of RhoA without co-transfection of wild-type Xdsh (Figure 7C, lane 2). The activation of RhoA induced by the stimulation of EphB2 with the ligand was inhibited by co-expression of Xdd1 (Figure 7C, lane 3). From these results, we concluded that Xdsh is involved in the activation of RhoA initiated by the activated EphB2 signaling. Dvl-2, which is most similar to Xdsh and is considered to be its mammalian counterpart, is expressed endogenously in 293T cells (data not shown). In our setting, ligand-stimulated EphB2 transduced signaling leading to RhoA activation via endogenous Dvl-2, and overexpressed Xdd1 competes with Dvl-2 for transduction of activated EphB2 signaling to RhoA. Next, we examined whether Xdsh also regulates RhoA activity in reverse signaling. The co-expression of wild-type Xdsh resulted in activation of RhoA in ephrin-B1-expressing cells when stimulated by EphB2 (Figure 7D, lanes 2 and 3). Interestingly, there was no activation of RhoA by co-expression of Xdsh when ephrin-B1 was not stimulated by EphB2 (Figure 7D, lane 4), even though the interaction between Xdsh and ephrin-B1 is constitutive (Figures 2C and 3A). This result implies that Xdsh mediates the reverse signaling initiated by EphB2-stimulated ephrin-B1 leading to the activation of RhoA.
Fig. 7. Xdsh mediates both forward and reverse signaling of EphB2 and ephrin-B1 leading to the activation of RhoA and Rho kinase. (A) 293T cells were transiently transfected with the mock plasmid (lane 1) or plasmids encoding myc-tagged Xdsh (lane 2). Cells were lysed and RhoA activation was examined in vivo by affinity precipitation of GTP-bound RhoA with a GST fusion protein of the RhoA-binding domain of Rho kinase. Bound proteins were immunoblotted (IB) with antibody for RhoA to detect activated endogenous RhoA. Expression of RhoA and Xdsh in each cell lysate is shown at the bottom (total lysate). (B) 293T cells were transiently transfected with the plasmids indicated above the lanes. Q61L RhoA is a constitutively activated mutant of RhoA. RhoA activation was examined in vivo as described in (A). The expression of RhoA and myc-tagged Dsh is shown on the bottom. (C) 293T cells stably expressing EphB2 were transfected with Nck and HA-tagged RhoA together with Xdd1 or mock plasmid, as indicated. Transfected cells were co-cultured with mock-transfected 293T cells (lane 1) or with 293T cells stably expressing ephrin-B1 (lanes 2 and 3) for 30 min before preparing the lysates. RhoA activation was examined as above except that anti-HA antibody was used to detect activation of transfected RhoA. (D) 293T cells stably expressing ephrin-B1 were transiently transfected with the plasmids indicated above the lanes. In lanes 2–4, 1 or 2 µg of wild-type Xdsh was transfected, as indicated. Transfected cells were co-cultured with 293T cells stably expressing EphB2 K661M (lanes 1–3) or mock-transfected 293T cells (lanes 4 and 5). RhoA activation was examined as above using anti-HA antibody. (E) 293T cells (lanes 1–6) or 293T cells stably expressing ephrin-B1 (lanes 7–10) were transfected with the plasmids as indicated above. Lanes 7–10: transfected cells were co-cultured with mock-transfected 293T cells (lanes 7 and 9) or 293T cells stably expressing EphB2 K661M (lanes 8 and 10) for 30 min. Cells were lysed for immunoprecipitation, and an in vitro kinase assay for Rho kinase was performed. The number at the bottom indicates the relative Rho kinase activity as fold activity present in cells lacking Xdsh (lanes 1 and 4). Similar results were obtained in three independent experiments. ‘Phospho-MBP’ indicates phosphorylated myelin basic protein. The expression of each protein in individual cell lysates (total lysate) was confirmed by immunoblotting at the bottom.
Next, we examined whether dsh mediates the activity of Rho-kinase (ROKα), which is a major effector protein of GTP-loaded RhoA. In the absence of EphB2 expression, ROKα was not activated adequately by overexpression of wild-type Xdsh (Figure 7E, compare lanes 1 and 2). In contrast, significantly enhanced ROKα activity was present in cells overexpressing EphB2 together with Nck and wild-type Xdsh (Figure 7E, lane 6). Moreover, we could show that stimulation of ephrin-B1 by co-culture with EphB2-expressing cells led to a slight activation of ROKα (Figure 7E, lane 8), which was enhanced significantly by the co-expression of Xdsh (Figure 7E, lane 10). These results indicate that Xdsh mediates both forward and reverse signaling of EphB2 and ephrin-B1 leading to the activation of RhoA and ROKα.
Xdsh mediates the patterning of the hindbrain in Xenopus laevis
In order to validate the commitment of Xdsh to the physiological roles in cell repulsion mediated by Eph and ephrin, we investigated whether Dsh mediates the patterning of hindbrain rhombomeres. The Eph family and their ligands are expressed in the alternate stripes in the presumptive hindbrain, and they mediate repulsive interactions between adjacent rhombomeres, which leads to the formation of sharp borders between them (Xu and Wilkinson, 1997).
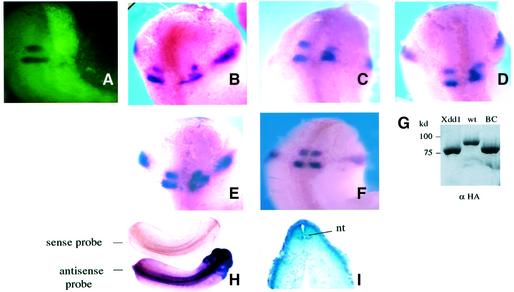
The RNA encoding Xdd1 was microinjected together with lineage tracer into one dorsal cell of four-cell stage embryos. Microinjection of Xdd1 mRNA into one hemisphere of the embryos facilitated internal comparison of any phenotype generated (Figure 7A). Taking advantage of the fact that Xkrox-20 expression was known to be sharply restricted to r3 and r5 by stage 20 (Xu et al., 1995), the XKrox-20 staining pattern was monitored in the early tailbud stage (stages 22–26). Overexpression of Xdd1 did not greatly affect the level of expression of the XKrox-20 gene itself, i.e. most embryos exhibited two Krox-20 staining stripes of almost the same intensity as on the uninjected side. Embryos showing marked reduction in XKrox-20 expression were eliminated from the evaluation of the shape of rhombomeres r3 and r5. An altered geometric pattern of XKrox-20 gene expression was observed in 29% of the embryos following Xdd1 mRNA microinjection (Figure 7B–E). Domains expressing XKrox-20 were irregular in shape, and r3/r5 expression domains moved toward the territory between them, typically resulting in fusion of r3 and r5 (Figure 7B–D) and failure to establish well-defined rhombomere boundaries. In some embryos, the rhombomeres were severely distorted and irregular (Figure 7E). An example of embryos having an almost normal XKrox-20 staining pattern in the rhombomere in spite of Xdd1 injection is shown in Figure 7F. On the other hand, as summarized in Table I, no alteration of r3/r5 was observed in any embryos microinjected with wild-type Xdsh. Co-injection of Xdsh-BC also did not significantly affect the XKrox-20 staining pattern. To address the question of whether endogenous Xdsh is involved in the hindbrain segmentation physiologically, whole-mount in situ hybridization was performed to investigate the localization of Xdsh mRNA during development. As shown in Figure 7H, Xdsh is expressed in the central nervous system, including the entire hindbrain region, throughout the early tailbud stage, but not in the notochord or somites, as confirmed in sections of stained embryos (Figure 7I). A similar distribution of Xdsh mRNA was observed in different stage embryos during the later stages, 18–25 (data not shown). These findings imply that Xdsh naturally plays a pivotal role in hindbrain segmentation.
Table I. Disruption of hindbrain in microinjected Xenopus embryos.
| Sample injected | No. affected/no. survivinga | No. of embryos injected |
|---|---|---|
| Xdd1 | 22/75 (29%) | 103 |
| Xdsh (wild type) | 0/48 (0%) | 50 |
| Xdsh-BC | 2/52 (3.8%) | 52 |
| Controlb | 0/84 (0%) | 84 |
aThe phenotypes of the injected embryos were scored by in situ hybridization at stages 22–26.
bIn the control, embryos were microinjected with lineage tracer alone.
Discussion
Examination of the association between Xdsh and EphB receptors and ephrin-B1 revealed that Xdsh forms a complex with EphB receptors via association with SH adaptors. We propose that there are two ways by which Xdsh may be recruited to ephrin-B1: either via the Xdsh–Grb4 complex to phosphorylated ephrin-B1, or by direct association. The regions of Xdsh and ephrin-B1 that are necessary for direct interaction between these two molecules should be identified in further studies. Although many proteins have been reported to be associated with dsh and to participate in the Wnt or Frz signaling cascades, the molecules that connect Dsh to tyrosine kinase receptors are unknown, except for EPS8. Mouse Dvl-1, an Xdsh protein homolog, is reported to associate with EPS8, a substrate for activated epidermal growth factor (EGF) receptor (Inobe et al., 1999), but the biological significance of this interaction is unclear. As Dsh binds directly to SH adaptors, a wider commitment of Dsh with tyrosine kinase receptors should be elucidated. It would be interesting to know whether Xdsh also associates either directly or indirectly with A-subclass ephrins and EphA receptors.
In vitro experiments using Xenopus animal explants revealed that co-expression of Xdd1 affected the cell sorting induced by EphB2 and ephrin-B1. There is a possibility that the expression of Xdd1 may have modified adhesion molecules and interfered indirectly with cell–cell adhesion/repulsion. However, dissociated cells co-expressing Xdd1 formed a spherical aggregate, the same as the cells without co-expression of Xdd1, and the time course of the appearance of these aggregates was not significantly different. There was no clear cell dissociation in Xdd1-injected aggregates. Moreover, in the re-aggregation assay, as shown in Figure 4, the cells expressing EphB2 or ephrin-B1 together with Xdd1 completely intermingled with those cells expressing only EphB2 or ephrin-B1 (data not shown). This is additional evidence that cell adhesion (cell affinity) itself is not significantly altered by expression of Xdd1. Although overexpression of ephrin-B1 in the animal region of Xenopus embryos is known to result in cell dissociation (Jones et al., 1998), we injected ephrin-B1 mRNA at a concentration that is not effective for cell dissociation, but high enough to be detectable in injected embryos. The restriction of the intermingling of the cells expressing Eph receptors and ephrins observed here is not the consequence of general inhibition of cell motility by Xdd1. Actually, chemotactic migration of 293T cells toward fibronectin is not affected by Xdd1 expression in Boyden chambers, as described in the Supplementary data, available at The EMBO Journal Online (data not shown).
Dsh is involved in two major signals: the canonical cascade and the PCP cascade. It mediates the PCP cascade via activation of RhoA and Rho kinase, and our observation that Xdsh mediates cell repulsion induced by Eph and ephrin is also closely related to RhoA activation. In view of the fact that Xdd1 is known to inhibit PCP signaling, including convergent and extension movements in Xenopus, the effect of co-expression of Xdd1 on hindbrain segmentation shown in Figure 8 may be due to inhibition of convergent and extension movements. However, if this defect in hindbrain segmentation were secondary to impaired convergence and extension, the number of affected embryos should have been much higher. As shown in Figure 8F, the dorsolaterally kinked phenotype, a supposed phenotype of defective convergence and extension, was observed in almost all Xdd1-injected embryos, including those without an obvious abnormality in the rhombomere structure. Moreover, overexpression of wild-type Xdsh has also been reported to inhibit convergence and extension movements, although the mechanism is not clearly understood (Wallingford et al., 2000). Our finding that wild-type Xdsh did not affect the XKrox20 expression pattern also contradicts the idea that the disruption of hindbrain segmentation is due to inhibition of convergence and extension. The possible dual role of Dsh in the PCP cascade and cell repulsion signaling can be elucidated in further experiments by construction of a preferential Xdsh mutant that inhibits Eph- and ephrin-mediated cell repulsion, but not the PCP pathway. Because the ΔDIX mutant did not affect the cell sorting shown in Figure 4G and H, it is apparent that the canonical cascade of Xdsh is not involved in the repulsive cell movement mediated by EphB2 and ephrin-B1. The canonical cascade from Wnt and Frz is reported to mediate the cell fate of the anterior–posterior neural axis. Microinjection of dominant-negative Wnt-8 resulted in the suppression of XKrox-20 expression (McGrew et al., 1995, 1997). However, microinjection of Xdsh-BC, which selectively blocks the canonical cascade, did not result in a significantly altered pattern of XKrox-20 staining in whole embryos, as shown in Table I, although some embryos showed weaker expression of XKrox-20 compared with the opposite control side. Therefore, we conclude that Xdd1 inhibits hindbrain segmentation not by interfering with the canonical or PCP cascade, but most probably by interfering with cell repulsion mediated by Eph and ephrin. It is also important to examine whether Xdsh may be involved in the regulation of Eph- and ephrin-mediated cell repulsion in other tissues or organs in a region- and time-specific manner, particularly in the regionally restricted migration of neural crest cells or axon guidance of neurons.
Fig. 8. Xdsh mediates Xenopus hindbrain segmenation. RNA encoding either Xdd1, wild-type Xdsh or Xdsh-BC tagged with HA was microinjected together with lineage tracer into one dorsal cell of four-cell stage embryos. (A) A microinjected embryo is shown in a dorsal view by fluorescence microscopy. (B–F) The embryos injected with Xdd1 were fixed at the early tailbud stage, and the XKrox-20 staining pattern was evaluated in a dorsal view by whole-mount in situ hybridization. (G) Expression of injected Xdsh in whole embryos is indicated by an immunoblot using anti-HA antibody. (H) Endogenous expression of Xdsh in stage 22 embryos is shown by whole-mount in situ hybridization. (I) A transverse section at the level of the hindbrain. nt, neural tube.
The mechanism of RhoA activation by Dsh is poorly understood. Because overexpression of Xdsh plus the N-terminal myristoylation signal derived from Src did not activate RhoA in the same assay (data not shown), the simple membrane localization of Xdsh does not activate RhoA effectively. It may be that an unidentified protein having the activity of a guanine nucleotide exchange factor (GEF) or a guanine nucleotide dissociation inhibitor (GDI) for RhoA also forms a complex with Dsh homologs and Eph receptors or ephrins in vivo. It is also interesting to examine whether the recently identified Formin homology protein Daam1, which forms a complex with Dvl and Rho, is also involved in the Xdsh-mediated RhoA activation in EphB2 or ephrin-B1 signaling (Habas et al., 2001). The finding that stimulation of ephrin-B1 by contact with EphB2-expressing cells induced activation of RhoA indicates a possibility that additional recruitment of Xdsh to phosphorylated ephrin-B1 by forming a complex with Grb4 may be required for the adequate activation of RhoA. It should also be clarified that the tyrosine phosphorylation of Xdsh is required for the activation of RhoA in EphB2- and ephrin-B1-mediated signaling. We further examined whether the interaction of EphB2 and ephrin-B1 also activated Rac1 and CDC42 by an affinity precipitation assay using the GST-tagged p21-binding domain of PAK1 (Otsuki et al., 2001). However, no activation of Rac1 and Cdc42 was detected in EphB2-overexpressing cells or in ephrin-B1-expressing cells stimulated by co-incubation with EphB2-expressing cells (data not shown). These results may be compatible with the idea that preferential activation of RhoA, but not the activation of Rac1 and Cdc42 in the restricted region of the cell membrane in contact with Eph and ephrin, is essential for the repulsive cell movement. Our observations verified the novel role of dsh in Eph–ephrin-induced signaling and a multifaced committment of this molecule in repulsive cell movements. This pivotal role in controlling the Eph–ephrin machinery implies that Dsh is an essential component of the principles of neural patterning and axon pathfinding during development.
Materials and methods
Plasmids and antibodies
Plasmids and antibodies used in these series of experiments are described in the Supplementary data.
Transfections, in vitro kinase assay and in vivo binding assays
Transfection of 293T human embryonal kidney cells was achieved by a calcium phosphate co-precipitation method with concurrent treatment with 25 µM chloroquine, essentially as described previously (Tanaka et al., 1995). For stimulation of EphB2 or ephrin-B1, transfected cells were co-cultured with 293T cells stably expressing ephrin-B1 or kinase domain-inactivated EphB2 (EphB2 K661M), respectively, for 30 min prior to lysis. For in vitro kinase assays of Rho kinase, cells were harvested for immunoprecipitation, and myelin basic protein (Sigma) was used as a substrate as previously described (Tanaka et al., 1995; Supplementary data).
In vitro binding assay
For in vitro binding assays, 35S-labeled full-length Xdsh and Xdd1 mutants were generated using a T7 reticulocyte lysate system (Promega). After binding 2.5 µg of GST-tagged proteins on glutathione–agarose to Xdsh proteins in TNGT (20 mM Tris pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X-100) for 1 h at 4°C, beads were washed five times with TNGT, and bound proteins were separated by SDS–PAGE.
Affinity precipitation
For affinity precipitation with GST–RB, 293T cells were lysed in lysis buffer [50 mM Tris–HCl pH 7.5, 500 mM NaCl, 10 mM MgCl2, 1 mM Na3VO4, 0.5% sodium deoxycholate, 1.0% Triton X-100, 5 µg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride (PMSF)]. Lysates were incubated with glutathione–Sepharose-conjugated GST–RB for 45 min at 4°C. Precipitates were washed four times in the washing buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 10 mM MgCl2, 1.0% Triton X-100), and the precipitated RhoA was detected by immunoblotting.
Embryos, microinjections, explant culture and whole-mount in situ hybridization
Fertilized embryos were prepared as described elsewhere (Newport and Kirschner, 1982). Capped synthetic mRNAs were generated by in vitro transcription of linearized pGHXP and pCS2+ vectors with the inserts as described (Tanaka et al., 1998). RNA was microinjected with or without fluorescent-conjugated dextran into embryos at the appropriate stage, as noted. For in situ hybridization, embryos were collected and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) overnight at 4°C. Whole-mount in situ hybridization was carried out with BM-purple AP substrate (Roche Molecular Biochemicals) according to a standard protocol. In some experiments, stained embryos were refixed in 4% paraformaldehyde for 30 min at room temperature, then embedded in plastic according to the manufacturer’s instructions (JB-4; Polysciences, Inc.), and sectioned at 10 µm.
Animal cap assays for cell intermingling
Two assays were performed to evaluate intermingling of EphB2-expressing cells and ephrin-B1-expressing cells in vitro. The details of these procedures are described in the Supplementary data.
Boyden chamber assay
Chemotactic cell migration experiments were performed with a Boyden chamber according to a standard protocol as described in the Supplementary data.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We are grateful to Drs N.Ikegaki (Children’s Hospital of Philadelphia), T.Pawson (Mount Sinai Hospital), R.Klein (European Molecular Biology Laboratory), S.Sokol (Harvard Medical Scool, Beth Israel Hospital), B.Mayer (University of Conncticut Health Center School of Medicine), L.Qullian (Indiana University School of Medicine) and K.Kaibuchi (Graduate School of Medicine, Nagoya University) for providing us with plasmids used in these experiments, Professor Terakawa and Dr Tsuboi (Laboratory of Cell Imaging, Photon Medical Resaech Center, Hamamatsu University School of Medicine) for assisting with the operation of the time-lapse microscope, and the entire staff of the Research Equipment Center at the Hamamatsu University School of Medicine for maintenance of the equipment. This work was supported by the Smoking Research Foundation, Grants-in-Aid for Cancer Research from the Ministry of Health and Welfare of Japan, and Grant-in Aid-for Scientific Research (B, 10470056) on priority areas (C-2,12218215; C-2, 13216044) and for Encouragement of Young Scientists (09770142) from the Ministry of Education, Culture, Sports Technology and Science of Japan.
References
- Bruckner K., Pasquale,E.B. and Klein,R. (1997) Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science, 275, 1640–1643. [DOI] [PubMed] [Google Scholar]
- Cowan C.A. and Henkemeyer,M. (2001) The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature, 413, 174–179. [DOI] [PubMed] [Google Scholar]
- Fanto M., Weber,U., Strutt,D.I. and Mlodzik,M. (2000) Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr. Biol., 10, 979–988. [DOI] [PubMed] [Google Scholar]
- Goodhill G.J. and Richards,L.J. (1999) Retinotectal maps: molecules, models and misplaced data. Trends Neurosci., 22, 529–534. [DOI] [PubMed] [Google Scholar]
- Habas R., Kato,Y. and He,X. (2001) Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell, 107, 843–854. [DOI] [PubMed] [Google Scholar]
- Heisenberg C.P., Tada,M., Rauch,G.J., Saude,L., Concha,M.L., Geisler,R., Stemple,D.L., Smith,J.C. and Wilson,S.W. (2000) Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature, 405, 76–81. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M., Orioli,D., Henderson,J.T., Saxton,T.M., Roder,J., Pawson,T. and Klein,R. (1996) Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell, 86, 35–46. [DOI] [PubMed] [Google Scholar]
- Holland S.J., Gale,N.W., Mbamalu,G., Yancopoulos,G.D., Henkemeyer,M. and Pawson,T. (1996) Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature, 383, 722–725. [DOI] [PubMed] [Google Scholar]
- Inobe M., Katsube,K., Miyagoe,Y., Nabeshima,Y. and Takeda,S. (1999) Identification of EPS8 as a Dvl1-associated molecule. Biochem. Biophys. Res. Commun., 266, 216–221. [DOI] [PubMed] [Google Scholar]
- Itoh K., Antipova,A., Ratcliffe,M.J. and Sokol,S. (2000) Interaction of dishevelled and Xenopus axin-related protein is required for wnt signal transduction. Mol. Cell. Biol., 20, 2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.L., Chong,L.D., Kim,J., Xu,R.H., Kung,H.F. and Daar,I.O. (1998) Loss of cell adhesion in Xenopus laevis embryos mediated by the cytoplasmic domain of XLerk, an erythropoietin-producing hepatocellular ligand. Proc. Natl Acad. Sci. USA, 95, 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K., Mather,N.K., Diella,F., Dottori,M., Boyd,A.W. and Klein,R. (2001) Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron, 29, 73–84. [DOI] [PubMed] [Google Scholar]
- Matsui T. et al. (1996) Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J., 15, 2208–2216. [PMC free article] [PubMed] [Google Scholar]
- McGrew L.L., Lai,C.J. and Moon,R.T. (1995) Specification of the anteriorposterior neural axis through synergistic interaction of the Wnt signaling cascade with noggin and follistatin. Dev. Biol., 172, 337–342. [DOI] [PubMed] [Google Scholar]
- McGrew L.L., Hoppler,S. and Moon,R.T. (1997) Wnt and FGF pathways cooperatively pattern anteriorposterior neural ectoderm in Xenopus. Mech. Dev., 69, 105–114. [DOI] [PubMed] [Google Scholar]
- Newport J. and Kirschner,M. (1982) A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell, 30, 687–696. [DOI] [PubMed] [Google Scholar]
- Orioli D. and Klein,R. (1997) The Eph receptor family: axonal guidance by contact repulsion. Trends Genet., 13, 354–359. [DOI] [PubMed] [Google Scholar]
- Orioli D., Henkemeyer,M., Lemke,G., Klein,R. and Pawson,T. (1996) Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. EMBO J., 15, 6035–6049. [PMC free article] [PubMed] [Google Scholar]
- Otsuki Y., Tanaka,M., Yoshii,S., Kawazoe,N., Nakaya, K and Sugimura,H. (2001) Tumor metastasis suppressor nm23H1 regulates Rac1 GTPase by interaction with Tiam1. Proc. Natl Acad. Sci. USA, 98, 4385–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale E.B. (1997) The Eph family of receptors. Curr. Opin. Cell Biol., 9, 608–615. [DOI] [PubMed] [Google Scholar]
- Sakanaka C., Sun,T.Q. and Williams,L.T. (2000) New steps in the Wnt/β-catenin signal transduction pathway. Recent Prog. Horm. Res., 55, 225–236. [PubMed] [Google Scholar]
- Shamah S.M. et al. (2001) EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell, 105, 233–244. [DOI] [PubMed] [Google Scholar]
- Sokol S.Y. (1996) Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol., 6, 1456–1467. [DOI] [PubMed] [Google Scholar]
- Sokol S.Y., Klingensmith,J., Perrimon,N. and Itoh,K. (1995) Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development, 121, 3487. [DOI] [PubMed] [Google Scholar]
- Stein E., Huynh-Do,U., Lane,A.A., Cerretti,D.P. and Daniel,T.O. (1998) Nck recruitment to Eph receptor, EphB1/ELK, couples ligand activation to c-Jun kinase. J. Biol. Chem., 273, 1303–1308. [DOI] [PubMed] [Google Scholar]
- Strutt D. (2001) Planar polarity: getting ready to ROCK. Curr. Biol., 11, R506–R509. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Gupta,R. and Mayer,B.J. (1995) Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol. Cell. Biol., 15, 6829–6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Wang,D.Y., Kamo,T., Igarashi,H., Wang,Y., Xiang,Y.Y., Tanioka,F., Naito,Y. and Sugimura,H. (1998) Interaction of EphB2-tyrosine kinase receptor and its ligand conveys dorsalization signal in Xenopus laevis development. Oncogene, 17, 1509–1516. [DOI] [PubMed] [Google Scholar]
- Wahl S., Barth,H., Ciossek,T., Aktories,K. and Mueller,B.K. (2000) Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol., 149, 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J.B. and Harland,R.M. (2001) Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development, 128, 2581–2592. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B., Rowning,B.A., Vogeli,K.M., Rothbacher,U., Fraser,S.E. and Harland,R.M. (2000) Dishevelled controls cell polarity during Xenopus gastrulation. Nature, 405, 81–85. [DOI] [PubMed] [Google Scholar]
- Winklbauer R., Medina,A., Swain,R.K. and Steinbeisser,H. (2001) Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature, 413, 856–860. [DOI] [PubMed] [Google Scholar]
- Winter C.G., Wang,B., Ballew,A., Royou,A., Karess,R., Axelrod,J.D. and Luo,L. (2001) Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell, 105, 81–91. [DOI] [PubMed] [Google Scholar]
- Xu Q. and Wilkinson,D.G. (1997) Eph-related receptors and their ligands: mediators of contact dependent cell interactions. J. Mol. Med., 75, 576–586. [DOI] [PubMed] [Google Scholar]
- Xu Q., Alldus,G., Holder,N. and Wilkinson,D.G. (1995) Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hindbrain. Development, 121, 4005–4016. [DOI] [PubMed] [Google Scholar]
- Xu Q., Mellitzer,G. and Wilkinson,D.G. (2000) Roles of Eph receptors and ephrins in segmental patterning. Philos. Trans. R. Soc. Lond. B Biol. Sci., 355, 993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]