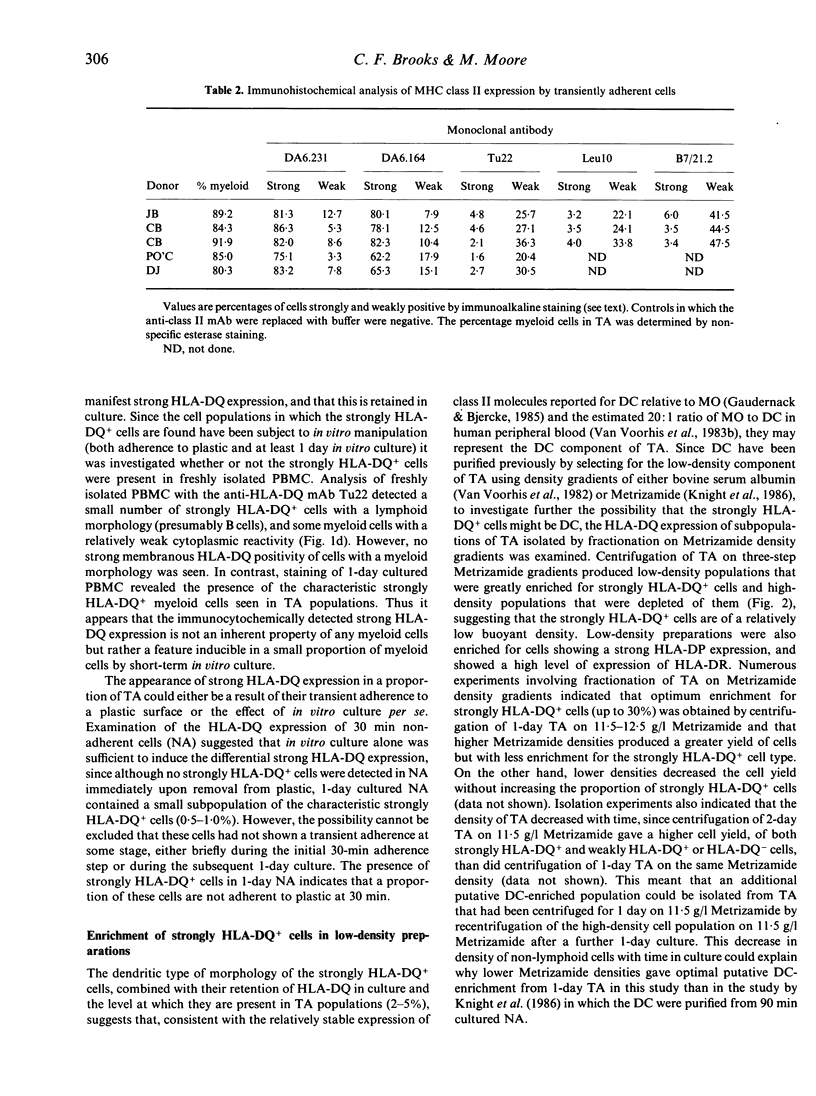
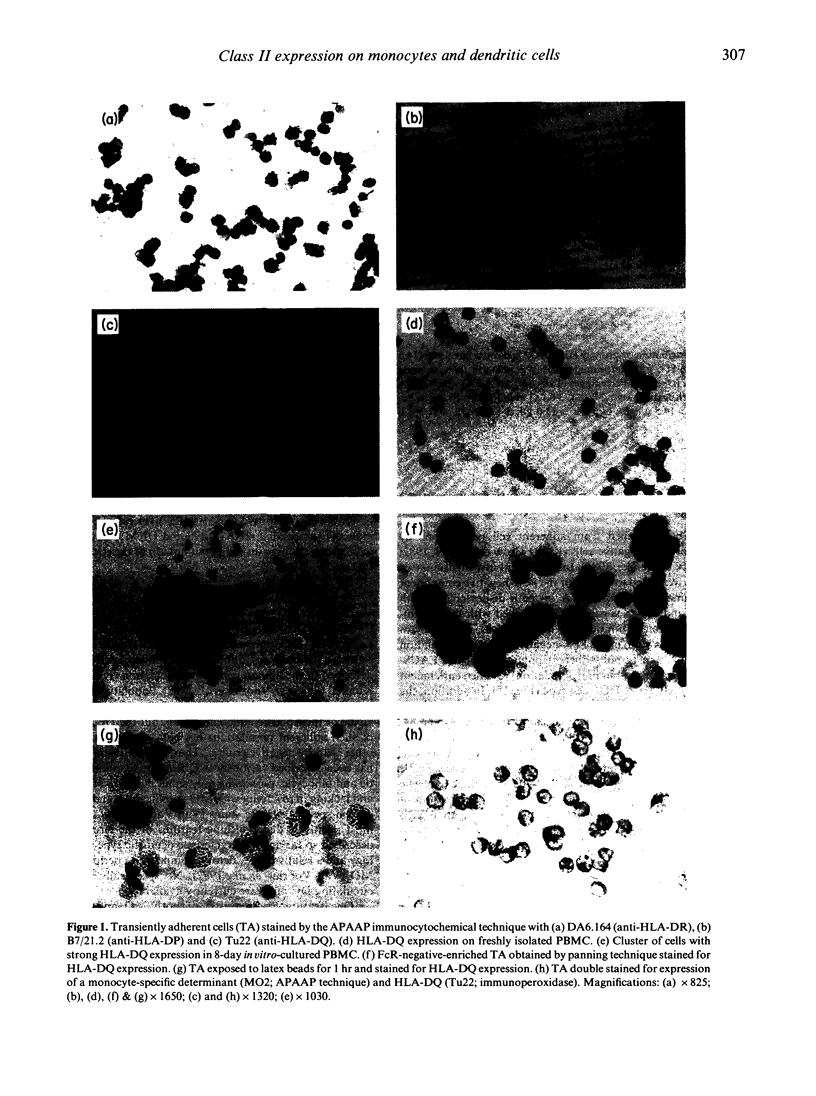
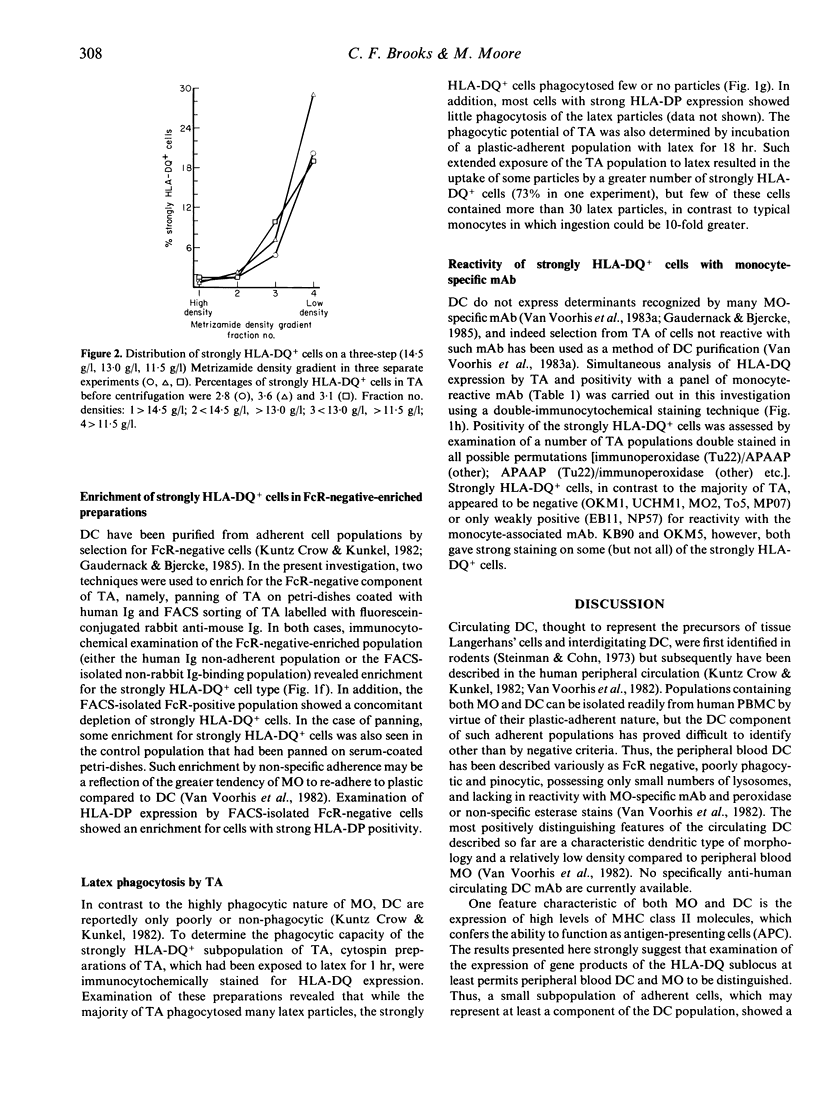
Abstract
Both monocytes (MO) and dendritic cells (DC) in human peripheral blood are of a plastic-adherent nature. The expression of the MHC class II sublocus products HLA-DP, -DQ and -DR on human peripheral blood transiently adherent cells (TA) was examined by an immunocytochemical staining technique. While most TA showed strong expression of molecules of the HLA-DR subtype, only a small proportion of cells (2-6%) showed strong HLA-DP or -DQ positivity. This strong expression of the HLA-DP and HLA-DQ sublocus products by a subset of TA was seen only after short-term culture; freshly isolated cells expressed comparatively low levels of these molecules. Enrichment for Fc receptor-negative or low-density cells from TA produced populations with strong HLA-DQ and -DP expression. Such co-enrichment of the strongly HLA-DQ+ and strongly HLA-DP+ cells suggests that the same cells express high levels of both types of MHC class II molecule. Immunocytochemical analysis of TA indicated that the strongly HLA-DQ+ cells, at least, were only weakly or non-reactive with the MO-specific monoclonal antibodies OKM1, UCHM1, MO2 and EB11. In addition, strongly HLA-DQ- or -DP-positive cells were poorly phagocytic in comparison with the majority of adherent cells. The apparent FcR-negative, low-density and weakly phagocytic nature of the strongly HLA-DQ/DP+ cells, combined with their lack of reactivity with several MO-specific antibodies, suggests that they may represent the DC component of TA. Such strong HLA-DQ/DP expression by DC may aid their positive identification in human peripheral blood and may be of relevance to DC function in antigen presentation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. C., Navarrete C., Solana R., Torres A., Pena J., Festenstein H. Differential expression of HLA-DR and HLA-DQ antigens on normal cells of the myelomonocytic lineage. Tissue Antigens. 1985 Nov;26(5):310–317. doi: 10.1111/j.1399-0039.1985.tb02229.x. [DOI] [PubMed] [Google Scholar]
- Balfour B. M., Drexhage H. A., Kamperdijk E. W., Hoefsmit E. C. Antigen-presenting cells, including Langerhans cells, veiled cells and interdigitating cells. Ciba Found Symp. 1981;84:281–301. doi: 10.1002/9780470720660.ch15. [DOI] [PubMed] [Google Scholar]
- Bjercke S., Gaudernack G. Dendritic cells and monocytes as accessory cells in T-cell responses in man. II. Function as antigen-presenting cells. Scand J Immunol. 1985 May;21(5):501–508. doi: 10.1111/j.1365-3083.1985.tb01839.x. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. HLA today. Hum Immunol. 1986 Dec;17(4):490–503. doi: 10.1016/0198-8859(86)90307-1. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Chen Y. X., Evans R. L., Pollack M. S., Lanier L. L., Phillips J. H., Rousso C., Warner N. L., Brodsky F. M. Characterization and expression of the HLA-DC antigens defined by anti-Leu 10. Hum Immunol. 1984 Aug;10(4):221–235. doi: 10.1016/0198-8859(84)90088-0. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Crow M. K., Kunkel H. G. Human dendritic cells: major stimulators of the autologous and allogeneic mixed leucocyte reactions. Clin Exp Immunol. 1982 Aug;49(2):338–346. [PMC free article] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984 Sep;38(3):293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Edwards J. A., Jones D. B., Evans P. R., Smith J. L. Differential expression of HLA class II antigens on human fetal and adult lymphocytes and macrophages. Immunology. 1985 Jul;55(3):489–500. [PMC free article] [PubMed] [Google Scholar]
- Fithian E., Kung P., Goldstein G., Rubenfeld M., Fenoglio C., Edelson R. Reactivity of Langerhans cells with hybridoma antibody. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2541–2544. doi: 10.1073/pnas.78.4.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin W. A., Mason D. Y., Pulford K., Falini B., Bliss E., Gatter K. C., Stein H., Clarke L. C., McGee J. O. Immunohistological analysis of human mononuclear phagocytes and dendritic cells by using monoclonal antibodies. Lab Invest. 1986 Mar;54(3):322–335. [PubMed] [Google Scholar]
- Gaudernack G., Bjercke S. Dendritic cells and monocytes as accessory cells in T-cell responses in man. I. Phenotypic analysis of dendritic cells and monocytes. Scand J Immunol. 1985 May;21(5):493–500. doi: 10.1111/j.1365-3083.1985.tb01838.x. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Naiem M., Mason D. Y., Stein H. Human complement (C3b) receptors defined by a mouse monoclonal antibody. Immunology. 1982 Apr;45(4):645–653. [PMC free article] [PubMed] [Google Scholar]
- Gonwa T. A., Frost J. P., Karr R. W. All human monocytes have the capability of expressing HLA-DQ and HLA-DP molecules upon stimulation with interferon-gamma. J Immunol. 1986 Jul 15;137(2):519–524. [PubMed] [Google Scholar]
- Gonwa T. A., Picker L. J., Raff H. V., Goyert S. M., Silver J., Stobo J. D. Antigen-presenting capabilities of human monocytes correlates with their expression of HLA-DS, an Ia determinant distinct from HLA-DR. J Immunol. 1983 Feb;130(2):706–711. [PubMed] [Google Scholar]
- Gonwa T. A., Stobo J. D. Differential expression of Ia molecules by human monocytes. J Clin Invest. 1984 Sep;74(3):859–866. doi: 10.1172/JCI111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy K., Van Heyningen V., Cohen B. B., Deane D. L., Steel C. M. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982 Nov;12(11):942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P. C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984 Dec;53(4):753–767. [PMC free article] [PubMed] [Google Scholar]
- Katz S. I., Tamaki K., Sachs D. H. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979 Nov 15;282(5736):324–326. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Bryant A., Edwards A. J., Burman S., Lever A., Clarke J., Webster A. D. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology. 1986 Apr;57(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Mertin J., Stackpoole A., Clark J. Induction of immune responses in vivo with small numbers of veiled (dendritic) cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6032–6035. doi: 10.1073/pnas.80.19.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald S. M., Pulford K., Falini B., Micklem K., Mason D. Y. A monoclonal antibody recognizing the p150/95 leucocyte differentiation antigen. Immunology. 1986 Nov;59(3):427–431. [PMC free article] [PubMed] [Google Scholar]
- Mayernik D. G., Ul-Haq A., Rinehart J. J. Differentiation-associated alteration in human monocyte-macrophage accessory cell function. J Immunol. 1983 May;130(5):2156–2160. [PubMed] [Google Scholar]
- Moreno J., Lipsky P. E. Functional heterogeneity of human antigen-presenting cells: presentation of soluble antigen but not self-Ia by monocytes. J Clin Immunol. 1986 Jan;6(1):9–20. doi: 10.1007/BF00915359. [DOI] [PubMed] [Google Scholar]
- Nuñez G., Giles R. C., Ball E. J., Hurley C. K., Capra J. D., Stastny P. Expression of HLA-DR, MB, MT and SB antigens on human mononuclear cells: identification of two phenotypically distinct monocyte populations. J Immunol. 1984 Sep;133(3):1300–1306. [PubMed] [Google Scholar]
- Patterson J. A., Edelson R. L. Interaction of T-lymphocytes with the epidermis: immunopathological studies. J Cutan Pathol. 1983 Dec;10(6):540–549. doi: 10.1111/j.1600-0560.1983.tb01505.x. [DOI] [PubMed] [Google Scholar]
- Rinehart J. J., Vessella R., Lange P., Kaplan M. E., Gormus B. J. Characterization and comparison of human monocyte- and macrophage-induced tumor cell cytotoxicity. J Lab Clin Med. 1979 Mar;93(3):361–369. [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Shen H. H., Talle M. A., Goldstein G., Chess L. Functional subsets of human monocytes defined by monoclonal antibodies: a distinct subset of monocytes contains the cells capable of inducing the autologous mixed lymphocyte culture. J Immunol. 1983 Feb;130(2):698–705. [PubMed] [Google Scholar]
- Silberberg-Sinakin I., Thorbecke G. J., Baer R. L., Rosenthal S. A., Berezowsky V. Antigen-bearing langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol. 1976 Aug;25(2):137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- Smith B. R., Ault K. A. Increase of surface Ia-like antigen expression on human monocytes independent of antigenic stimuli. J Immunol. 1981 Nov;127(5):2020–2027. [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Lustig D. S., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J Exp Med. 1974 Jun 1;139(6):1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Nussenzweig M. C. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–147. doi: 10.1111/j.1600-065x.1980.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Sztein M. B., Steeg P. S., Johnson H. M., Oppenheim J. J. Regulation of human peripheral blood monocyte DR antigen expression in vitro by lymphokines and recombinant interferons. J Clin Invest. 1984 Feb;73(2):556–565. doi: 10.1172/JCI111243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Valinsky J., Hoffman E., Luban J., Hair L. S., Steinman R. M. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983 Jul 1;158(1):174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A. J., DeMars R., Trowbridge I. S., Bach F. H. Detection of a novel human class II HLA antigen. 1983 Jul 28-Aug 3Nature. 304(5924):358–361. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Wang C. Y., Halper J., Hoffman T. Studies with B-cell allo- and hetero-antisera: parallel reactivity and special properties. Scand J Immunol. 1976;5(6-7):745–757. doi: 10.1111/j.1365-3083.1976.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- van Heyningen V., Guy K., Newman R., Steel C. M. Human MHC class II molecules as differentiation markers. Immunogenetics. 1982;16(5):459–469. doi: 10.1007/BF00372104. [DOI] [PubMed] [Google Scholar]