Abstract
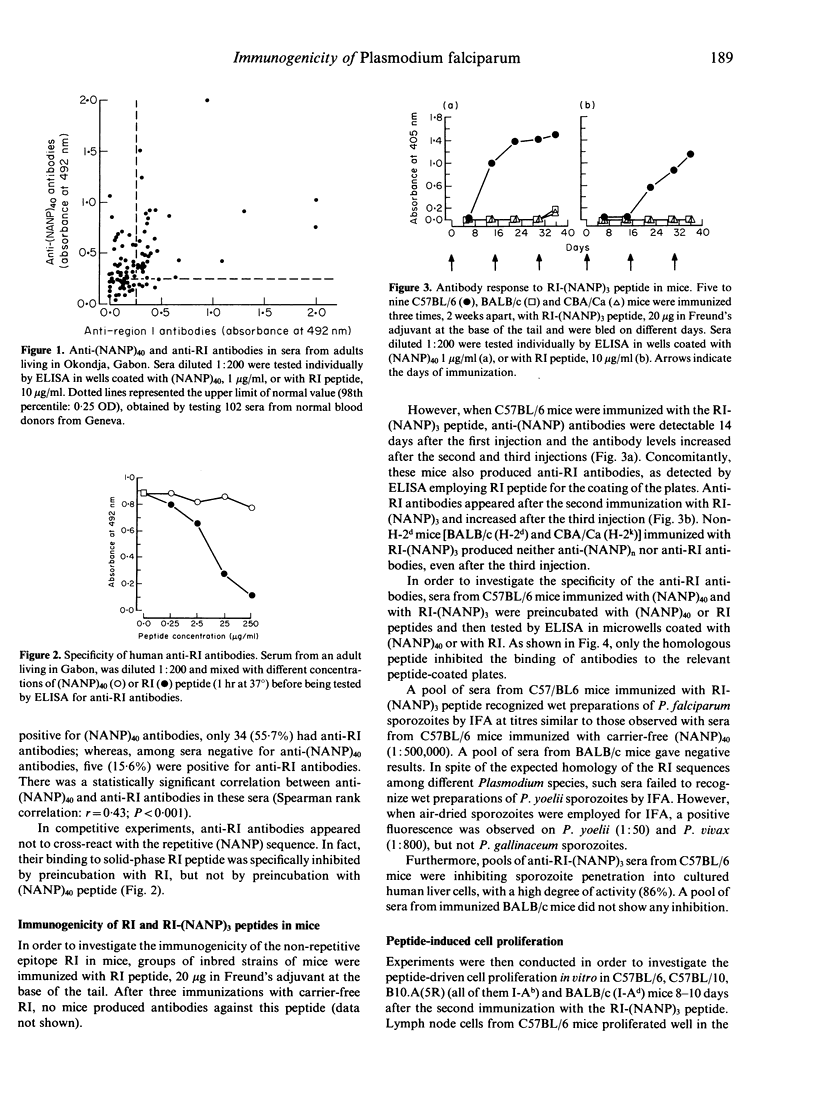
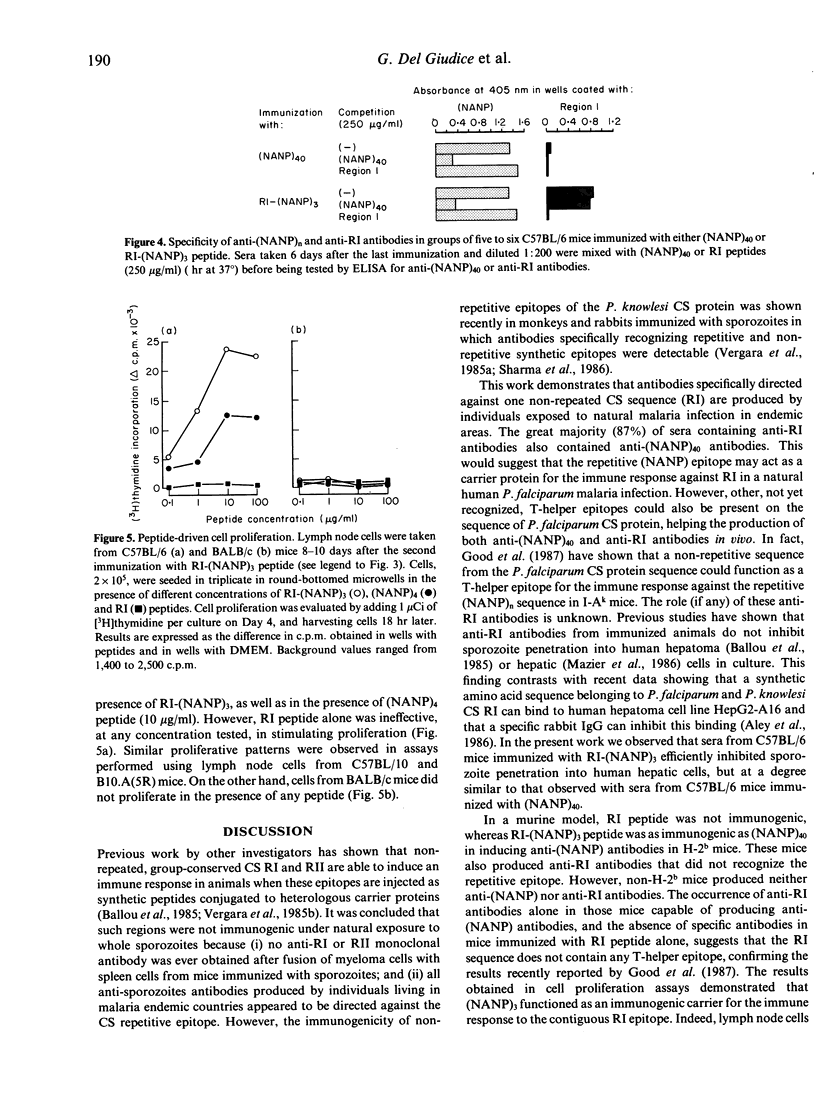
In the present work, the hypothesis that individuals naturally exposed to Plasmodium falciparum malaria infection in endemic areas produce antibodies directed against non-repetitive epitopes of the circumsporozoite protein was investigated. Using a synthetic peptide reproducing the non-repetitive group-conserved region I sequence, we have shown that specific anti-region I antibodies are detectable in sera from endemic countries. Of these sera, 87% also had antibodies against the immunodominant repetitive epitope (Asn-Ala-Asn-Pro, NANP) of P. falciparum. In order to study the immunogenicity of this non-repetitive epitope, a synthetic peptide consisting of both region I and three (NANP) repeats [RI-(NANP)3] was used to immunize inbred strains of mice. H-2b mice produced antibodies against both the repetitive and the non-repetitive epitope. These antibodies were specific for each epitope, recognized P. falciparum sporozoites in immunofluorescence, and inhibited sporozoite penetration into human liver cells in vitro. Non-H-2b mice were completely unresponsive. Lymph node cells from H-2b mice immunized with RI-(NANP)3 peptide proliferated in the presence of RI-(NANP)3 and of (NANP)4 peptide, but never in the presence of RI peptide alone. These findings demonstrate that in the configuration used (i) the non-repetitive epitope region I does not carry T-helper epitopes; (ii) the (NANP) repetitive epitope may act as a carrier for the immune response to region I in mice; and (iii) therefore, immune response to region I in man probably depends on the recognition of T-cell epitopes similar to those involved in the anti-NANP response: i.e. such a T epitope may be NANP itself in responding individuals or another, not yet recognized, sporozoite T-cell epitope.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aley S. B., Bates M. D., Tam J. P., Hollingdale M. R. Synthetic peptides from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium knowlesi recognize the human hepatoma cell line HepG2-A16 in vitro. J Exp Med. 1986 Dec 1;164(6):1915–1922. doi: 10.1084/jem.164.6.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Cooper J. A., Merino J., Verdini A. S., Pessi A., Togna A. R., Engers H. D., Corradin G., Lambert P. H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986 Nov 1;137(9):2952–2955. [PubMed] [Google Scholar]
- Del Giudice G., Engers H. D., Tougne C., Biro S. S., Weiss N., Verdini A. S., Pessi A., Degremont A. A., Freyvogel T. A., Lambert P. H. Antibodies to the repetitive epitope of Plasmodium falciparum circumsporozoite protein in a rural Tanzanian community: a longitudinal study of 132 children. Am J Trop Med Hyg. 1987 Mar;36(2):203–212. doi: 10.4269/ajtmh.1987.36.203. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Verdini A. S., Pinori M., Pessi A., Verhave J. P., Tougne C., Ivanoff B., Lambert P. H., Engers H. D. Detection of human antibodies against Plasmodium falciparum sporozoites using synthetic peptides. J Clin Microbiol. 1987 Jan;25(1):91–96. doi: 10.1128/jcm.25.1.91-96.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druilhe P., Pradier O., Marc J. P., Miltgen F., Mazier D., Parent G. Levels of antibodies to Plasmodium falciparum sporozoite surface antigens reflect malaria transmission rates and are persistent in the absence of reinfection. Infect Immun. 1986 Aug;53(2):393–397. doi: 10.1128/iai.53.2.393-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger D. J., Arnot D. E., Tam J. P., Nussenzweig V., Enea V. Circumsporozoite protein of Plasmodium berghei: gene cloning and identification of the immunodominant epitopes. Mol Cell Biol. 1986 Nov;6(11):3965–3972. doi: 10.1128/mcb.6.11.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinski M. R., Arnot D. E., Cochrane A. H., Barnwell J. W., Nussenzweig R. S., Enea V. The circumsporozoite gene of the Plasmodium cynomolgi complex. Cell. 1987 Jan 30;48(2):311–319. doi: 10.1016/0092-8674(87)90434-x. [DOI] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Maloy W. L., Lunde M. N., Margalit H., Cornette J. L., Smith G. L., Moss B., Miller L. H., Berzofsky J. A. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science. 1987 Feb 27;235(4792):1059–1062. doi: 10.1126/science.2434994. [DOI] [PubMed] [Google Scholar]
- Lockyer M. J., Schwarz R. T. Strain variation in the circumsporozoite protein gene of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Jan 2;22(1):101–108. doi: 10.1016/0166-6851(87)90073-9. [DOI] [PubMed] [Google Scholar]
- Mazier D., Mellouk S., Beaudoin R. L., Texier B., Druilhe P., Hockmeyer W., Trosper J., Paul C., Charoenvit Y., Young J. Effect of antibodies to recombinant and synthetic peptides on P. falciparum sporozoites in vitro. Science. 1986 Jan 10;231(4734):156–159. doi: 10.1126/science.3510455. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Lal A. A., de la Cruz V. F., Miller L. H., Maloy W. L., Charoenvit Y., Beaudoin R. L., Guerry P., Wistar R., Jr, Hoffman S. L. Sequence of the immunodominant epitope for the surface protein on sporozoites of Plasmodium vivax. Science. 1985 Dec 20;230(4732):1381–1383. doi: 10.1126/science.2416057. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Ozaki L. S., Svec P., Nussenzweig R. S., Nussenzweig V., Godson G. N. Structure of the plasmodium knowlesi gene coding for the circumsporozoite protein. Cell. 1983 Oct;34(3):815–822. doi: 10.1016/0092-8674(83)90538-x. [DOI] [PubMed] [Google Scholar]
- Sharma S., Gwadz R. W., Schlesinger D. H., Godson G. N. Immunogenicity of the repetitive and nonrepetitive peptide regions of the divergent CS protein of Plasmodium knowlesi. J Immunol. 1986 Jul 1;137(1):357–361. [PubMed] [Google Scholar]
- Togna A. R., Del Giudice G., Verdini A. S., Bonelli F., Pessi A., Engers H. D., Corradin G. Synthetic Plasmodium falciparum circumsporozoite peptides elicit heterogenous L3T4+ T cell proliferative responses in H-2b mice. J Immunol. 1986 Nov 1;137(9):2956–2960. [PubMed] [Google Scholar]
- Vergara U., Gwadz R., Schlesinger D., Nussenzweig V., Ferreira A. Multiple non-repeated epitopes on the circumsporozoite protein of Plasmodium knowlesi. Mol Biochem Parasitol. 1985 Mar;14(3):283–292. doi: 10.1016/0166-6851(85)90056-8. [DOI] [PubMed] [Google Scholar]
- Vergara U., Ruiz A., Ferreira A., Nussenzweig R. S., Nussenzweig V. Conserved group-specific epitopes of the circumsporozoite proteins revealed by antibodies to synthetic peptides. J Immunol. 1985 May;134(5):3445–3448. [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]


