Abstract
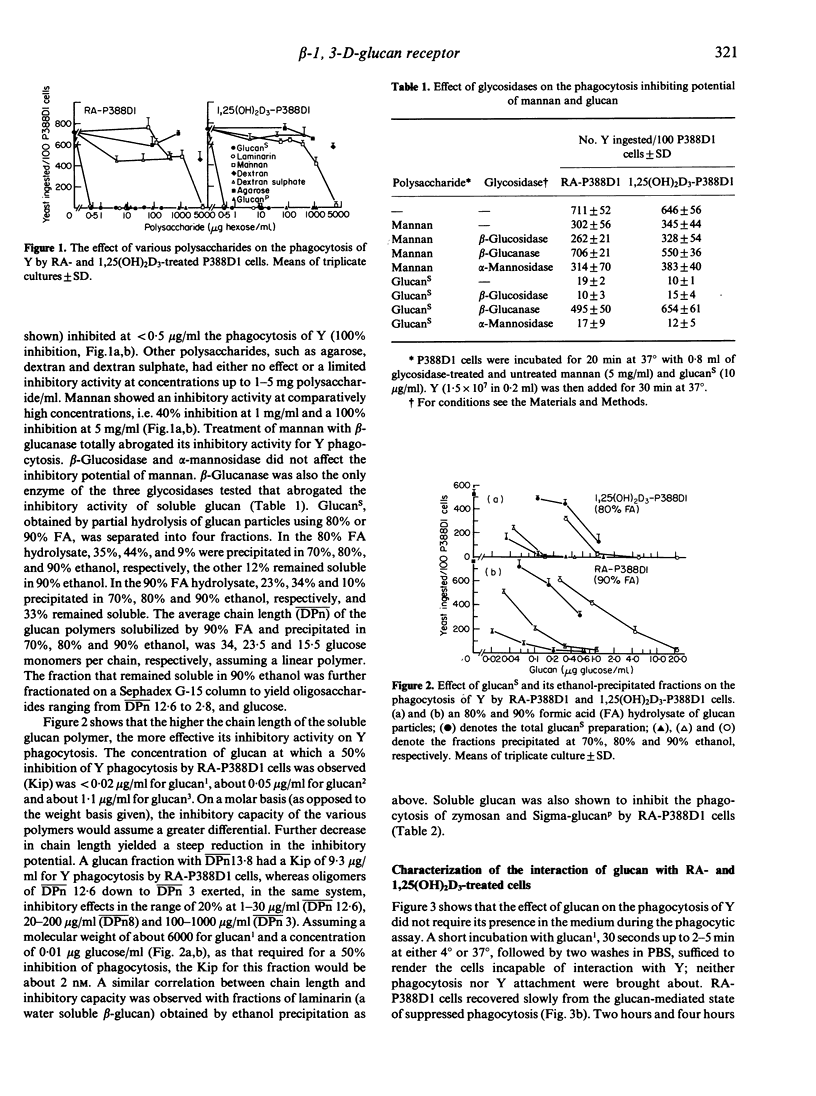
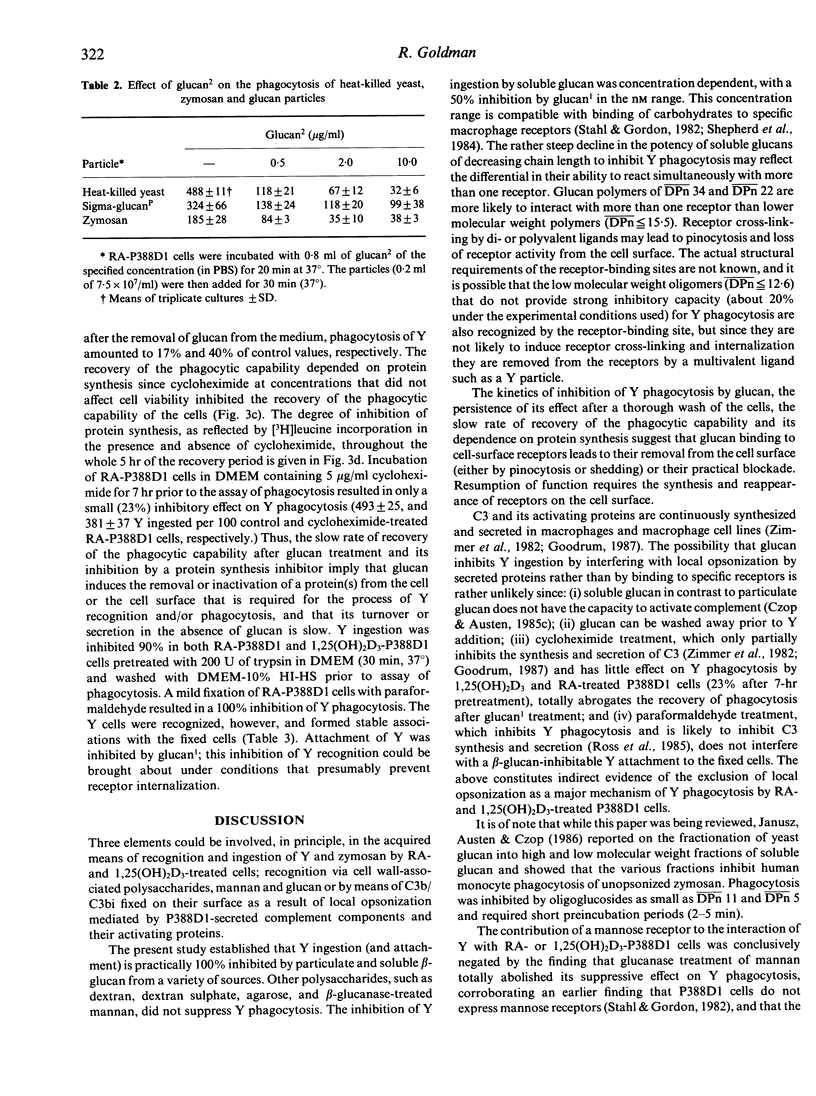
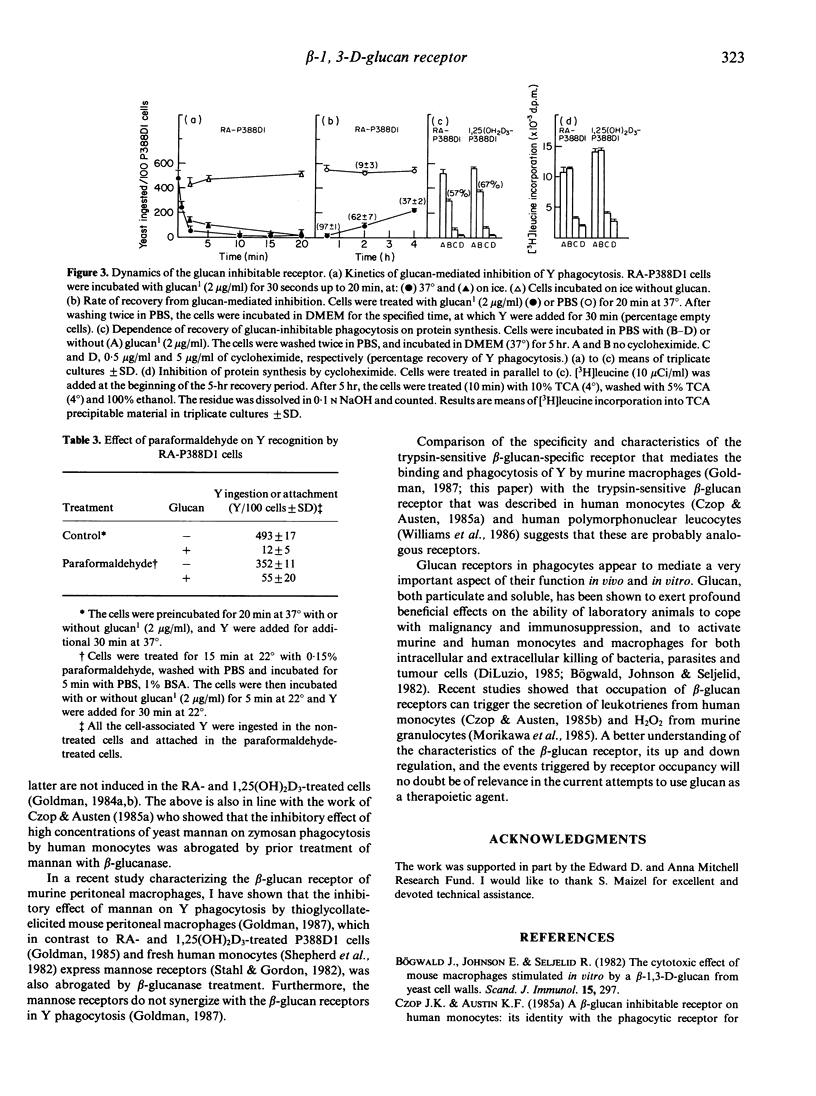
Retinoic acid (RA) and 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) induce the capability to phagocytose heat-killed yeast (Y) (Saccharomyces cerevisiae) in P388D1 cells. Y phagocytosis is specifically inhibited (100%) by particulate and soluble beta-1,3-D-glucan. Other polysaccharides, such as agarose, dextran and dextran sulphate, are not inhibitory. The inhibitory capacity of mannan was totally abrogated by treatment with beta-glucanase, suggesting that its activity is derived from a residual beta-glucan structure. Partial hydrolysis of glucan particles with formic acid yielded soluble glucan that was fractionated according to size. Glucan1, glucan2 and glucan3 had an average chain length of 34, 23.5 and 15.5 glucose units, respectively. Fifty percent inhibition of Y phagocytosis by RA-P388D1 cells was attained at less than 0.02 microgram/ml (approximately 2 nM) glucan1 and at 1.1 micrograms/ml glucan3. A further decrease in chain length (less than or equal to 12.6) resulted in oligomers of marginal inhibitory activity. Preincubation of RA- and 1,25(OH)2D3-P388D1 cells with glucan1 for 30 seconds to 5 min, at 4 degrees or 37 degrees, followed by washes with buffer, sufficed to bring about 85-95% inhibition of Y phagocytosis. Recovery of the phagocytic capability was time dependent and required protein synthesis, suggesting a glucan1-induced removal of membrane receptors. The results suggest that recognition and ingestion of Y by RA- or 1,25(OH)2D3-treated P388D1 cells depends almost exclusively on a beta-glucan-specific receptor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bögwald J., Johnson E., Seljelid R. The cytotoxic effect of mouse macrophages stimulated in vitro by a beta-1,3-D-glucan from yeast cell walls. Scand J Immunol. 1982 Mar;15(3):297–304. doi: 10.1111/j.1365-3083.1982.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. A beta-glucan inhibitable receptor on human monocytes: its identity with the phagocytic receptor for particulate activators of the alternative complement pathway. J Immunol. 1985 Apr;134(4):2588–2593. [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. Generation of leukotrienes by human monocytes upon stimulation of their beta-glucan receptor during phagocytosis. Proc Natl Acad Sci U S A. 1985 May;82(9):2751–2755. doi: 10.1073/pnas.82.9.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. Properties of glycans that activate the human alternative complement pathway and interact with the human monocyte beta-glucan receptor. J Immunol. 1985 Nov;135(5):3388–3393. [PubMed] [Google Scholar]
- DI CARLO F. J., FIORE J. V. On the composition of zymosan. Science. 1958 Apr 4;127(3301):756–757. doi: 10.1126/science.127.3301.756-a. [DOI] [PubMed] [Google Scholar]
- Di Luzio N. R. Update on the immunomodulating activities of glucans. Springer Semin Immunopathol. 1985;8(4):387–400. doi: 10.1007/BF01857392. [DOI] [PubMed] [Google Scholar]
- Di Luzio N. R., Williams D. L., McNamee R. B., Edwards B. F., Kitahama A. Comparative tumor-inhibitory and anti-bacterial activity of soluble and particulate glucan. Int J Cancer. 1979 Dec 15;24(6):773–779. doi: 10.1002/ijc.2910240613. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., Hill M., Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984 Jan 1;159(1):244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. Effect of retinoic acid on the proliferation and phagocytic capability of murine macrophage-like cell lines. J Cell Physiol. 1984 Jul;120(1):91–102. doi: 10.1002/jcp.1041200113. [DOI] [PubMed] [Google Scholar]
- Goldman R. Induction of a high phagocytic capability in P388D1, a macrophage-like tumor cell line, by 1 alpha, 25-dihydroxyvitamin D3. Cancer Res. 1984 Jan;44(1):11–19. [PubMed] [Google Scholar]
- Goldman R. Synergism and antagonism in the effects of 1 alpha,25-dihydroxyvitamin D3, retinoic acid, dexamethasone, and a tumor-promoting phorbol ester on the functional capability of P388D1 cells: phagocytosis and transglutaminase activity. Cancer Res. 1985 Jul;45(7):3118–3124. [PubMed] [Google Scholar]
- Goodrum K. J. Complement component C3 secretion by mouse macrophage-like cell lines. J Leukoc Biol. 1987 Apr;41(4):295–301. doi: 10.1002/jlb.41.4.295. [DOI] [PubMed] [Google Scholar]
- Janusz M. J., Austen K. F., Czop J. K. Isolation of soluble yeast beta-glucans that inhibit human monocyte phagocytosis mediated by beta-glucan receptors. J Immunol. 1986 Nov 15;137(10):3270–3276. [PubMed] [Google Scholar]
- Johnson E., Bøgwald J., Eskeland T., Seljelid R. Complement (C3) receptor-mediated attachment of agarose beads to mouse peritoneal macrophages and human monocytes. Scand J Immunol. 1983 May;17(5):403–410. doi: 10.1111/j.1365-3083.1983.tb00806.x. [DOI] [PubMed] [Google Scholar]
- Morikawa K., Kamegaya S., Yamazaki M., Mizuno D. Hydrogen peroxide as a tumoricidal mediator of murine polymorphonuclear leukocytes induced by a linear beta-1,3-D-glucan and some other immunomodulators. Cancer Res. 1985 Aug;45(8):3482–3486. [PubMed] [Google Scholar]
- Newman S. L., Mikus L. K. Deposition of C3b and iC3b onto particulate activators of the human complement system. Quantitation with monoclonal antibodies to human C3. J Exp Med. 1985 Jun 1;161(6):1414–1431. doi: 10.1084/jem.161.6.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Sasaki T., Takasuka N., Chihara G., Maeda Y. Y. Antitumor activity of degraded products of lentinan: its correlation with molecular weight. Gan. 1976 Apr;67(2):191–195. [PubMed] [Google Scholar]
- Shepherd V. L., Campbell E. J., Senior R. M., Stahl P. D. Characterization of the mannose/fucose receptor on human mononuclear phagocytes. J Reticuloendothel Soc. 1982 Dec;32(6):423–431. [PubMed] [Google Scholar]
- Shepherd V. L., Freeze H. H., Miller A. L., Stahl P. D. Identification of mannose 6-phosphate receptors in rabbit alveolar macrophages. J Biol Chem. 1984 Feb 25;259(4):2257–2261. [PubMed] [Google Scholar]
- Speert D. P., Silverstein S. C. Phagocytosis of unopsonized zymosan by human monocyte-derived macrophages: maturation and inhibition by mannan. J Leukoc Biol. 1985 Nov;38(5):655–658. doi: 10.1002/jlb.38.5.655. [DOI] [PubMed] [Google Scholar]
- Stahl P., Gordon S. Expression of a mannosyl-fucosyl receptor for endocytosis on cultured primary macrophages and their hybrids. J Cell Biol. 1982 Apr;93(1):49–56. doi: 10.1083/jcb.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983 Jan;96(1):160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Topley N., Alobaidi H. M., Harber M. J. Activation of human polymorphonuclear leucocytes by particulate zymosan is related to both its major carbohydrate components: glucan and mannan. Immunology. 1986 May;58(1):117–124. [PMC free article] [PubMed] [Google Scholar]
- Zimmer B., Hartung H. P., Scharfenberger G., Bitter-Suermann D., Hadding U. Quantitative studies of the secretion of complement component C3 by resident, elicited and activated macrophages. Comparison with C2, C4 and lysosomal enzyme release. Eur J Immunol. 1982 May;12(5):426–430. doi: 10.1002/eji.1830120513. [DOI] [PubMed] [Google Scholar]
- van Furth R., van Schadewijk-Nieuwstad M., Elzenga-Claasen I., Cornelisse C., Nibbering P. Morphological, cytochemical, functional, and proliferative characteristics of four murine macrophage-like cell lines. Cell Immunol. 1985 Feb;90(2):339–357. doi: 10.1016/0008-8749(85)90199-6. [DOI] [PubMed] [Google Scholar]


