Abstract
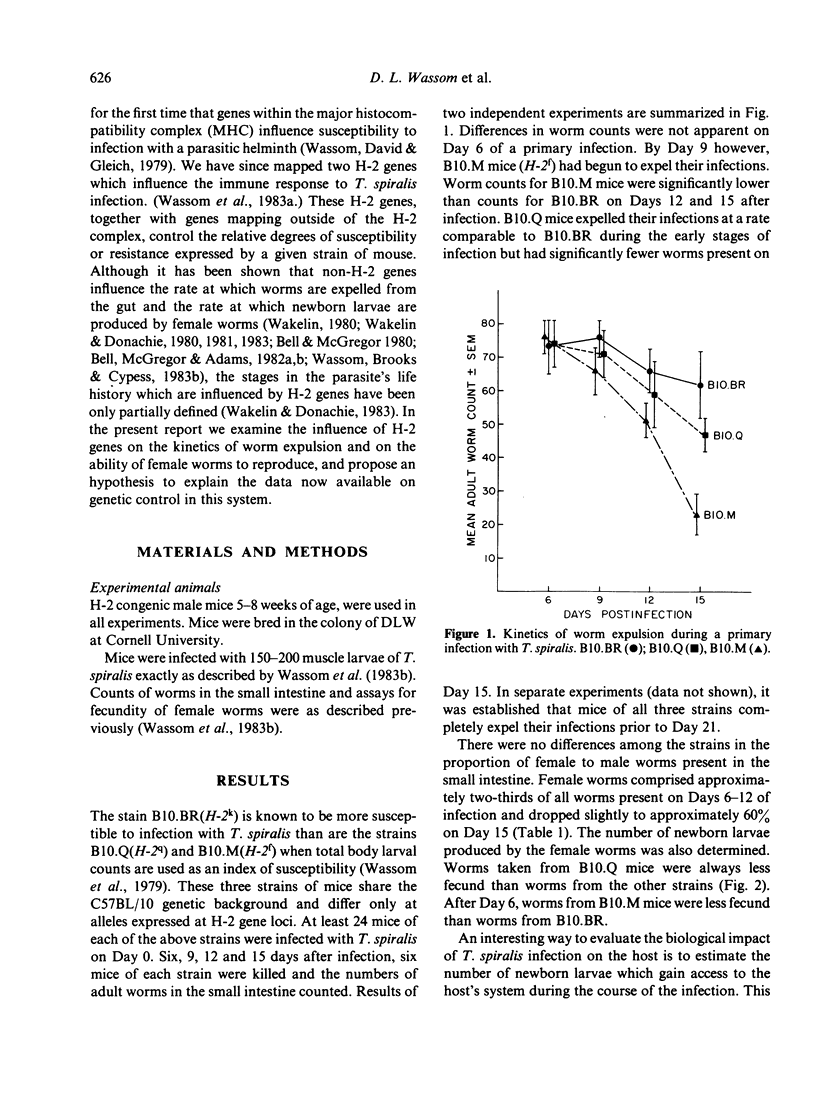
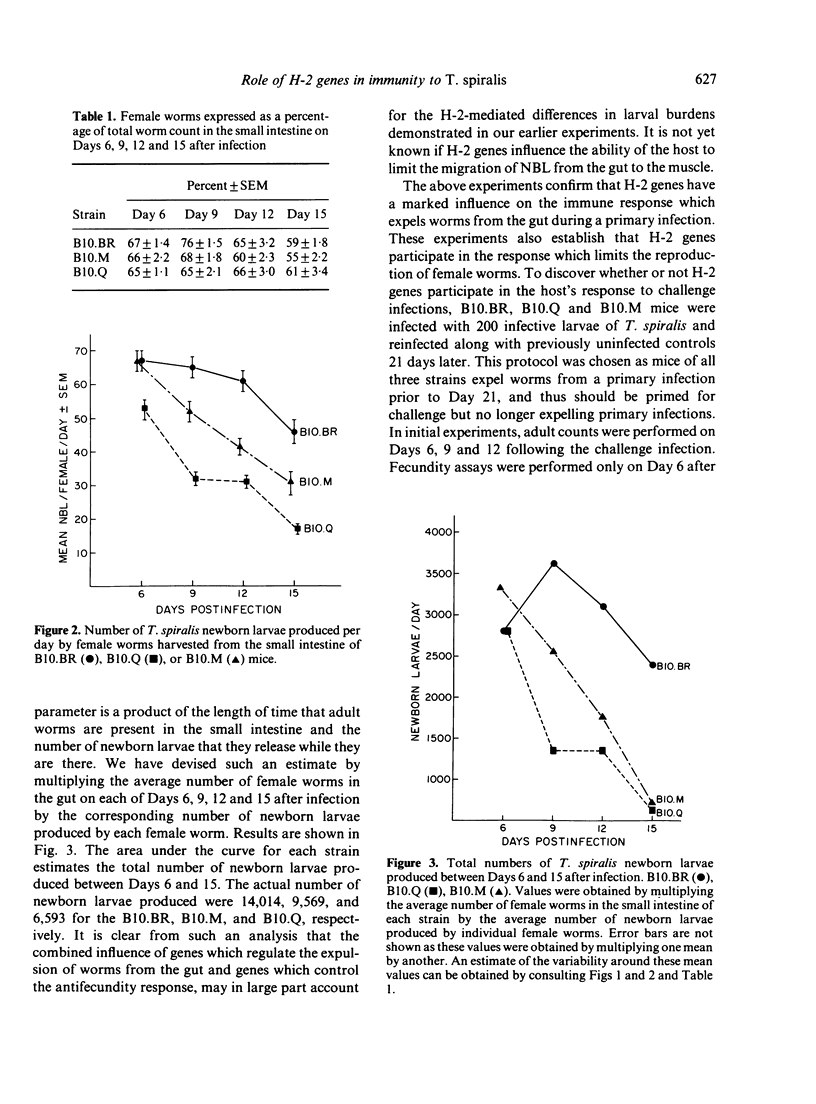
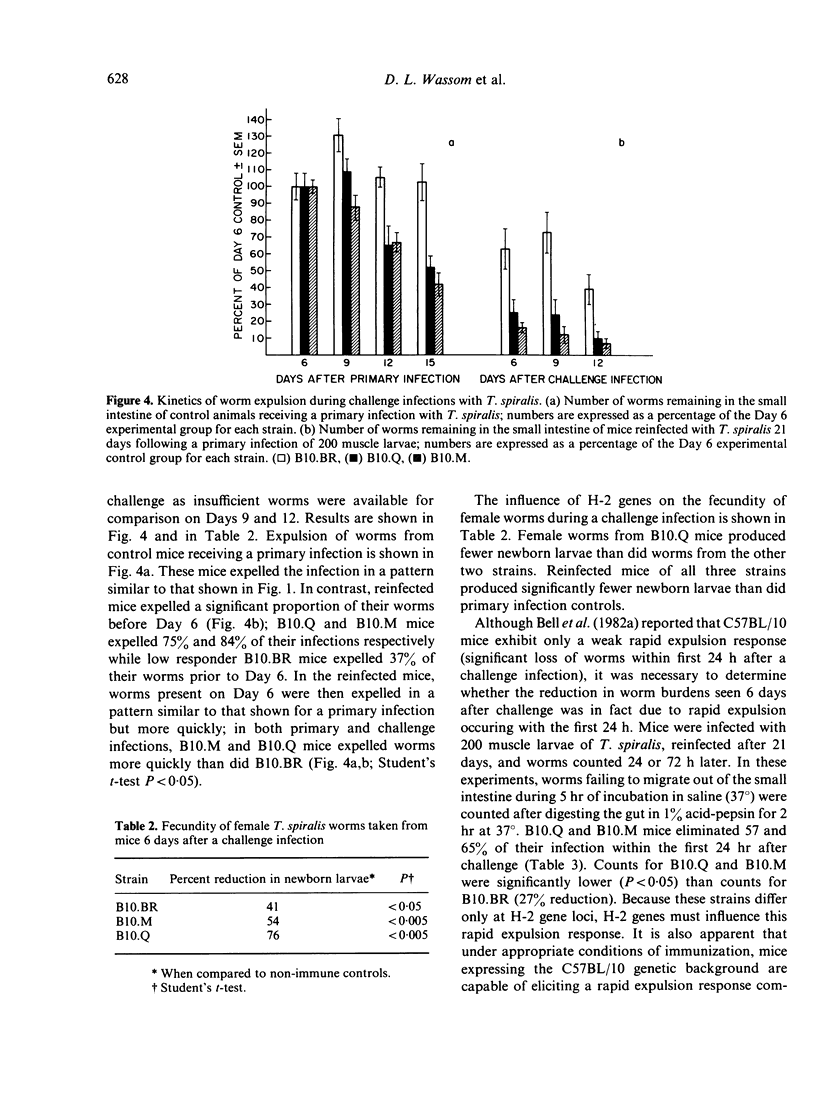
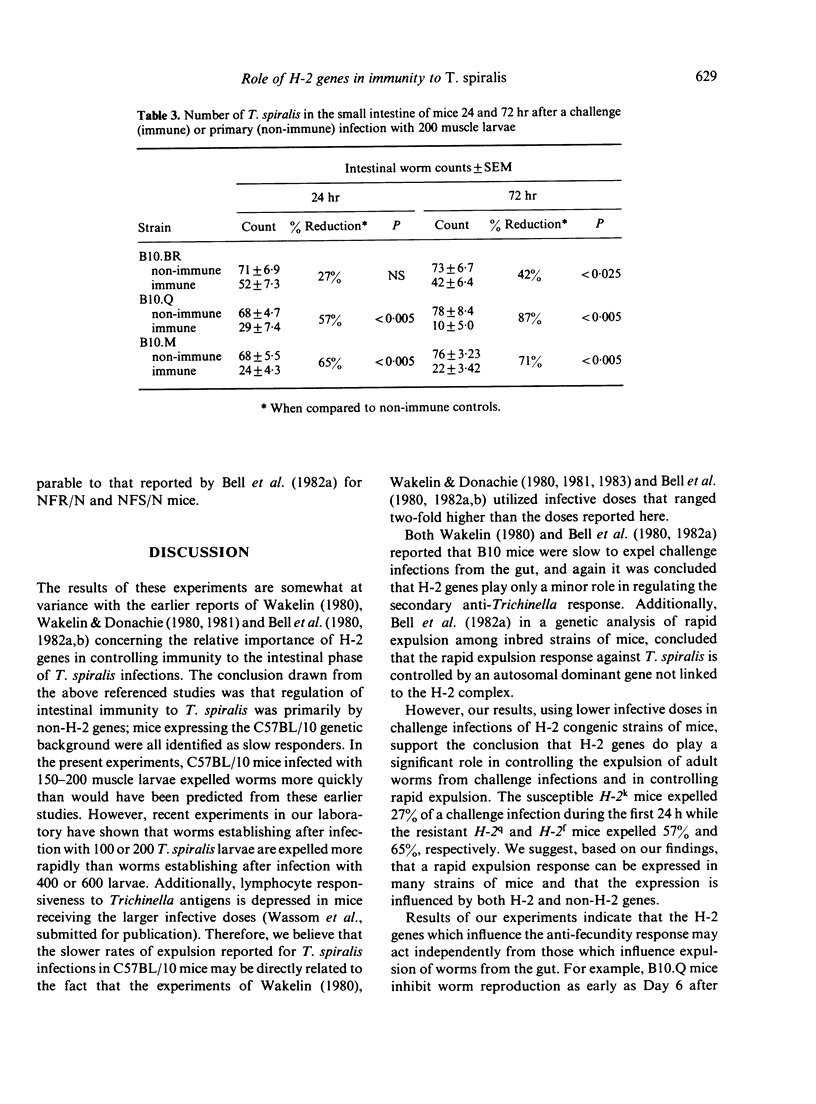
H-2 congenic strains of mice were compared for their ability to expel T. spiralis infections from the small intestine and for their ability to limit the reproduction of adult female worms. B10.M mice (H-2f) expelled both primary and challenge infections more quickly than did the strains B10.Q(H-2q) and B10.BR(H-2k). During a primary infection, expulsion of worms from B10.M mice began before Day 9 post-infection and worm counts differed significantly (P less than .05 Student's t-test) from counts in B10.BR mice on each of Days 12 and 15.B10.Q mice expelled worms more rapidly than B10.BR but worm counts did not differ significantly until Day 15. Whereas B10.M mice responded most quickly to expel worms from the gut, B10.Q mice were most effective in limiting worm reproduction. Female worms harvested from B10.Q mice and cultured for 24 hr in vitro produced significantly fewer newborn larvae than did worms from B10.M or B10.BR mice. Worms from B10.M mice were less fecund than worms from B10.BR, but this difference was not apparent before Day 9 post-infection, and worms from B10.M were always more fecund than worms from B10.Q. Challenge infections 21 days following a priming dose of 200 T. spiralis muscle larvae were rejected very quickly. B10.M mice expelled 65% of their worms during the first 24 h. By Day 6 after challenge, B10.M mice had expelled 84% of their worms; B10.Q and B10.BR expelled 75% and 37% respectively. These results suggest that a rapid expulsion response may be expressed in many different strains of mice depending on how the mice are immunized and the size of the infecting dose. Fecundity of female worms 6 days following a challenge infection was reduced for all strains tested when compared to primary infection controls; however, worms from B10.Q mice were less fecund than worms from B10.M or B10.BR. Results of these experiments demonstrate that H-2 genes play an important role in controlling the immune response which expels worms from the gut and the response which limits worm reproduction. These H-2-controlled differences are expressed during both primary and challenge infections. As the present results conflict somewhat with results published elsewhere, we have proposed a new hypothesis to explain the data collected in our laboratories thus far. According to this hypothesis, the anti-adult response, the anti-fecundity response, and the rapid expulsion response are under independent genetic control and influenced by the interacting products of both H-2 and non-H-2 genes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. G., McGregor D. D., Adams L. S. Trichinella spiralis: characterization and strain distribution of rapid expulsion in inbred mice. Exp Parasitol. 1982 Jun;53(3):301–314. doi: 10.1016/0014-4894(82)90073-x. [DOI] [PubMed] [Google Scholar]
- Bell R. G., McGregor D. D., Adams L. S. Trichinella spiralis: genetic basis for differential expression of phase-specific intestinal immunity in inbred mice. Exp Parasitol. 1982 Jun;53(3):315–325. doi: 10.1016/0014-4894(82)90074-1. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Watanabe D. H., Strebel F. R., McBride R. A. Complementing MHC- and non-MHC-linked genes and resistance to avian sarcoma virus-induced tumours in inbred lines of chickens. J Immunogenet. 1981 Jun;8(3):215–223. doi: 10.1111/j.1744-313x.1981.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Krco C. J., David C. S., Wassom D. L. Characterization of an in vitro proliferation response to solubilized Trichinella spiralis antigens: role of la antigens and Ly-1+ T cells. Cell Immunol. 1982 Apr;68(2):359–367. doi: 10.1016/0008-8749(82)90120-4. [DOI] [PubMed] [Google Scholar]
- Strassmann G., Eshhar Z., Mozes E. Genetic regulation of delayed-type hypersensitivity responses to poly(LTyr,LGlu)-poly(DLAla)--poly(LLys). II. Evidence for a T-T-cell collaboration in delayed-type hypersensitivity responses and for a T-cell defect at the efferent phase in nonresponder H-2k mice. J Exp Med. 1980 Mar 1;151(3):628–636. doi: 10.1084/jem.151.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of immunity to Trichinella spiralis. Donor bone marrow cells determine responses to infection in mouse radiation chimaeras. Immunology. 1981 Aug;43(4):787–792. [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of immunity to Trichinella spiralis: influence of H-2-linked genes on immunity to the intestinal phase of infection. Immunology. 1983 Feb;48(2):343–350. [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of immunity to parasites: adoptive transfer of immunity between inbred strains of mice characterized by rapid and slow immune expulsion of Trichinella spiralis. Parasite Immunol. 1980 Winter;2(4):249–260. doi: 10.1111/j.1365-3024.1980.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Wassom D. L., Brooks B. O., Babish J. G., David C. S. A gene mapping between the S and D regions of the H-2 complex influences resistance to Trichinella spiralis infections of mice. J Immunogenet. 1983 Oct;10(5):371–378. doi: 10.1111/j.1744-313x.1983.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Wassom D. L., Brooks B. O., Cypess R. H., David C. S. Trichinella spiralis: role of non-H-2 genes in resistance to primary infection in mice. Exp Parasitol. 1983 Apr;55(2):153–158. doi: 10.1016/0014-4894(83)90009-7. [DOI] [PubMed] [Google Scholar]


