Abstract
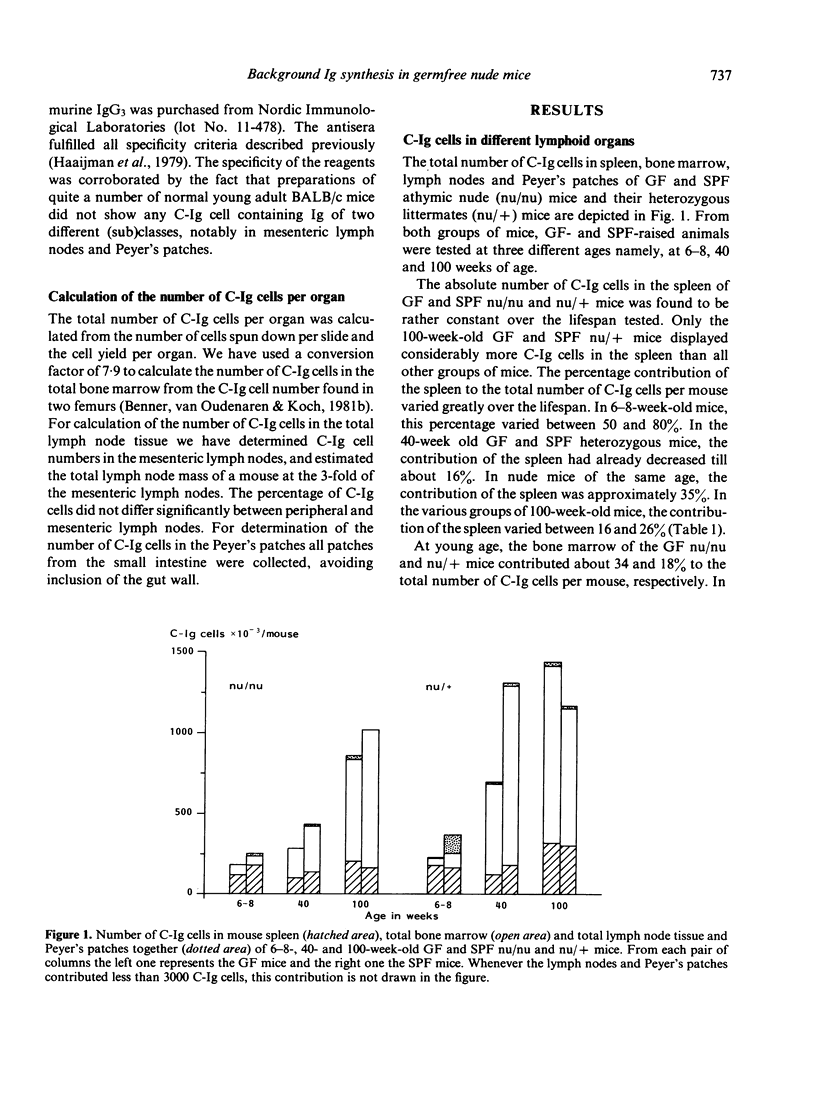
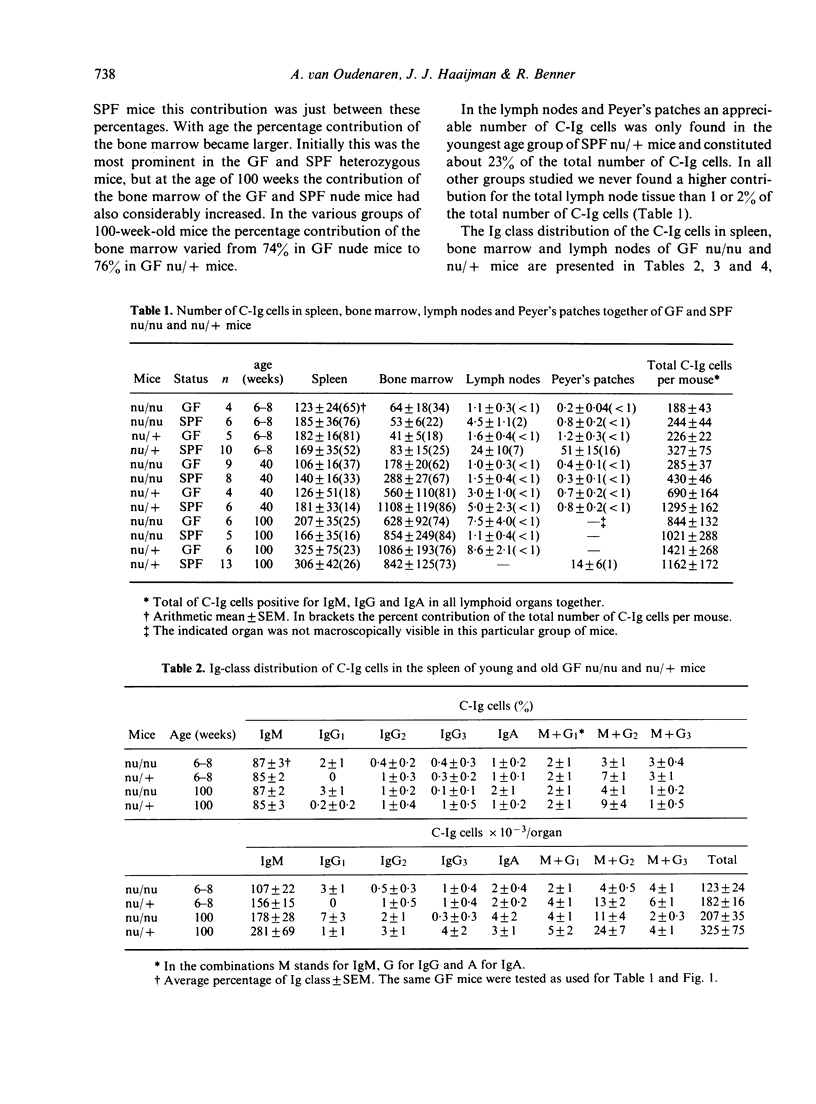
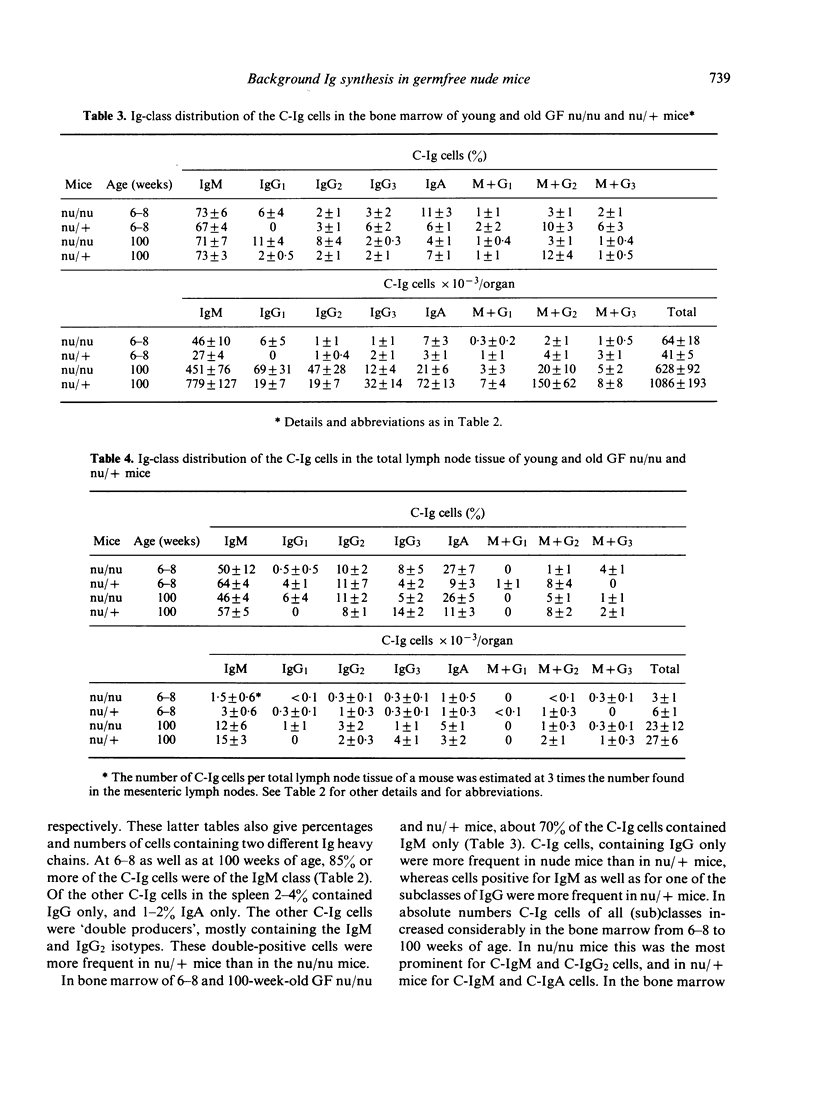
The distribution of background Ig-secreting cells, measured as cells containing cytoplasmic immunoglobulin (C-Ig cells), over spleen, bone marrow, lymph nodes and Peyer's patches was studied in congenitally athymic (nude) mice and heterozygous euthymic mice as a function of age and immune status (germ-free (GF) vs specific pathogen-free (SPF]. In young athymic as well as in young euthymic mice, the spleen was found to contain the great part of all C-Ig cells, irrespective of whether the mice were GF or SPF. The number of C-Ig cells in the spleen was found to be rather constant over the life span, while the number of C-Ig cells in the bone marrow of all groups of mice greatly increased with age. This indicates that the relative shift of C-Ig cells to the bone marrow is neither dependent on the presence of the thymus, nor on the microbiological status of the mice. However, at young and intermediate age the microbiological status of the mice did affect the total number of C-Ig cells per mouse. This was mainly due to the effect upon the bone marrow, mesenteric lymph nodes and Peyer's patches. At these ages the background Ig synthesis in these organs appeared to be mainly dependent on external antigenic stimulation, in contrast to the spleen, where the Ig synthesis appeared to be mainly due to endogenous stimulation. The Ig (sub)class distribution of the C-Ig cells was different for all different organs tested. Hardly or no difference in percentage distribution was found between the GF nude and GF heterozygous mice. Most C-Ig cells in spleen, bone marrow and lymph nodes of the GF mice were of the IgM isotype. C-IgG and C-IgA cells occurred in substantial percentages only in bone marrow and lymph nodes. In the lymph nodes of GF nude mice a remarkably high percentage of C-IgA cells was found.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Cooper M. D., Kearney J. F., Lawton A. R., Parkhouse R. M. Sequential expression of immunoglobulin on developing mouse B lymphocytes: a systematic survey that suggests a model for the generation of immunoglobulin isotype diversity. J Immunol. 1978 Jun;120(6):2041–2049. [PubMed] [Google Scholar]
- Bankhurst A. D., Lambert P. H., Miescher P. A. Studies on the thymic dependence of the immunoglobulin classes of the mouse (38570). Proc Soc Exp Biol Med. 1975 Feb;148(2):501–504. doi: 10.3181/00379727-148-38570. [DOI] [PubMed] [Google Scholar]
- Benner R., Haaijman J. J. Aging of the lymphoid system at the organ level. With special reference to the bone marrow as site of antibody production. Dev Comp Immunol. 1980 Fall;4(4):591–603. doi: 10.1016/s0145-305x(80)80062-0. [DOI] [PubMed] [Google Scholar]
- Benner R., van Oudenaren A. Antibody formation in mouse bone marrow. IV. The influence of splenectomy on the bone marrow plaque-forming cell response to sheep red blood cells. Cell Immunol. 1975 Oct;19(2):167–182. doi: 10.1016/0008-8749(75)90201-4. [DOI] [PubMed] [Google Scholar]
- Benner R., van Oudenaren A., Haaijman J. J. Deficient antibody formation in the bone marrow of nude mice. Immunology. 1978 Oct;35(4):619–626. [PMC free article] [PubMed] [Google Scholar]
- Benner R., van Oudenaren A., Haaijman J. J., Slingerland-Teunissen J., Wostmann B. S., Hijmans W. Regulation of the "spontaneous' (background) immunoglobulin synthesis. Int Arch Allergy Appl Immunol. 1981;66(4):404–415. doi: 10.1159/000232849. [DOI] [PubMed] [Google Scholar]
- Benner R., van Oudenaren A., de Ruiter H. Antibody formation in mouse bone marrow. IX. Peripheral lymphoid organs are involved in the initiation of bone marrow antibody formation. Cell Immunol. 1977 Nov;34(1):125–137. doi: 10.1016/0008-8749(77)90235-0. [DOI] [PubMed] [Google Scholar]
- Cosenza H. Detection of anti-idiotype reactive cells in the response to phosphorylcholine. Eur J Immunol. 1976 Feb;6(2):114–116. doi: 10.1002/eji.1830060208. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Benner R., Björklund M., Forni L., Holmberg D., Ivars F., Martinez-A C., Pettersson S. A "trans" perspective on the control of immunoglobulin c gene expression. Immunol Rev. 1982;67:87–114. doi: 10.1111/j.1600-065x.1982.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Heremans J. F. The distribution of immunoglobulin-containing cells along the human gastrointestinal tract. Gastroenterology. 1966 Sep;51(3):305–316. [PubMed] [Google Scholar]
- Fahey J. L., Barth W. F., Law L. W. Normal immunoglobulins and antibody response in neonatally thymectomized mice. J Natl Cancer Inst. 1965 Oct;35(4):663–678. [PubMed] [Google Scholar]
- Haaijman J. J., Schuit H. R., Hijmans W. Immunoglobulin-containing cells in different lymphoid organs of the CBA mouse during its life-span. Immunology. 1977 Apr;32(4):427–434. [PMC free article] [PubMed] [Google Scholar]
- Haaijman J. J., Slingerland-Teunissen J., Benner R., Van Oudenaren A. The distribution of cytoplasmic immunoglobulin containing cells over various lymphoid organs of congenitally athymic (nude) mice as a function of age. Immunology. 1979 Feb;36(2):271–278. [PMC free article] [PubMed] [Google Scholar]
- Hijmans W., Schuit H. R., Klein F. An immunofluorescence procedure for the detection of intracellular immunoglobulins. Clin Exp Immunol. 1969 Apr;4(4):457–472. [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Osmond D. G., Julius M. H., Benner R. The mechanism of thymus-dependent antibody formation in bone marrow. J Immunol. 1981 Apr;126(4):1447–1451. [PubMed] [Google Scholar]
- Lawrence E. C., Arnaud-Battandier F., Koski I. R., Dooley N. J., Muchmore A. V., Blaese R. M. Tissue distribution of immunoglobulin-secreting cells in normal and IgA-deficient chickens. J Immunol. 1979 Oct;123(4):1767–1771. [PubMed] [Google Scholar]
- Lawton A. R., 3rd, Cooper M. D. Modification of B lymphocyte differentiation by anti-immunoglobulins. Contemp Top Immunobiol. 1974;3:193–225. doi: 10.1007/978-1-4684-3045-5_8. [DOI] [PubMed] [Google Scholar]
- Martinez-Alonso C., Coutinho A., Augustin A. A. Immunoglobulin C-gene expression. I. The commitment to IgG subclass of secretory cells is determined by the quality of the nonspecific stimuli. Eur J Immunol. 1980 Sep;10(9):698–702. doi: 10.1002/eji.1830100908. [DOI] [PubMed] [Google Scholar]
- Porter P., Noakes D. E., Allen W. D. Intestinal secretion of immunoglobulins in the preruminant calf. Immunology. 1972 Sep;23(3):299–312. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Megson M., Owen J. J., Cooper M. D. Early production of intracellular IgM by B-lymphocyte precursors in mouse. Nature. 1976 Jan 22;259(5540):224–226. doi: 10.1038/259224a0. [DOI] [PubMed] [Google Scholar]
- Steele E. J., Cunningham A. J. High proportion of Ig-producing cells making autoantibody in normal mice. Nature. 1978 Aug 3;274(5670):483–484. doi: 10.1038/274483a0. [DOI] [PubMed] [Google Scholar]
- Taylor R. B., Wortis H. H. Thymus dependence of antibody response: variation with dose of antigen and class of antibody. Nature. 1968 Nov 30;220(5170):927–928. doi: 10.1038/220927a0. [DOI] [PubMed] [Google Scholar]
- Turesson I. Distribution of immunoglobulin-containing cells in human bone marrow and lymphoid tissues. Acta Med Scand. 1976;199(4):293–304. doi: 10.1111/j.0954-6820.1976.tb06735.x. [DOI] [PubMed] [Google Scholar]
- Vossen J. M., Langlois van den Bergh R. The detection of cytoplasmic immunoglobulins by immunofluorescence: improvements in techniques and standardization procedures. J Immunol Methods. 1976;13(1):71–82. doi: 10.1016/0022-1759(76)90188-5. [DOI] [PubMed] [Google Scholar]
- Wostmann B. S. Nutrition and metabolism of the germfree mammal. World Rev Nutr Diet. 1975;22:40–92. doi: 10.1159/000397975. [DOI] [PubMed] [Google Scholar]
- van Muiswinkel W. B., van Soest P. L. Thymus dependence of the IgA response to sheep erythrocytes. Immunology. 1975 Feb;28(2):287–291. [PMC free article] [PubMed] [Google Scholar]
- van Snick J. L., Masson P. L. Incidence and specificities of IgA and IgM anti-AgG autoantibodies in various mouse strains and colonies. J Exp Med. 1980 Jan 1;151(1):45–55. doi: 10.1084/jem.151.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


