Abstract
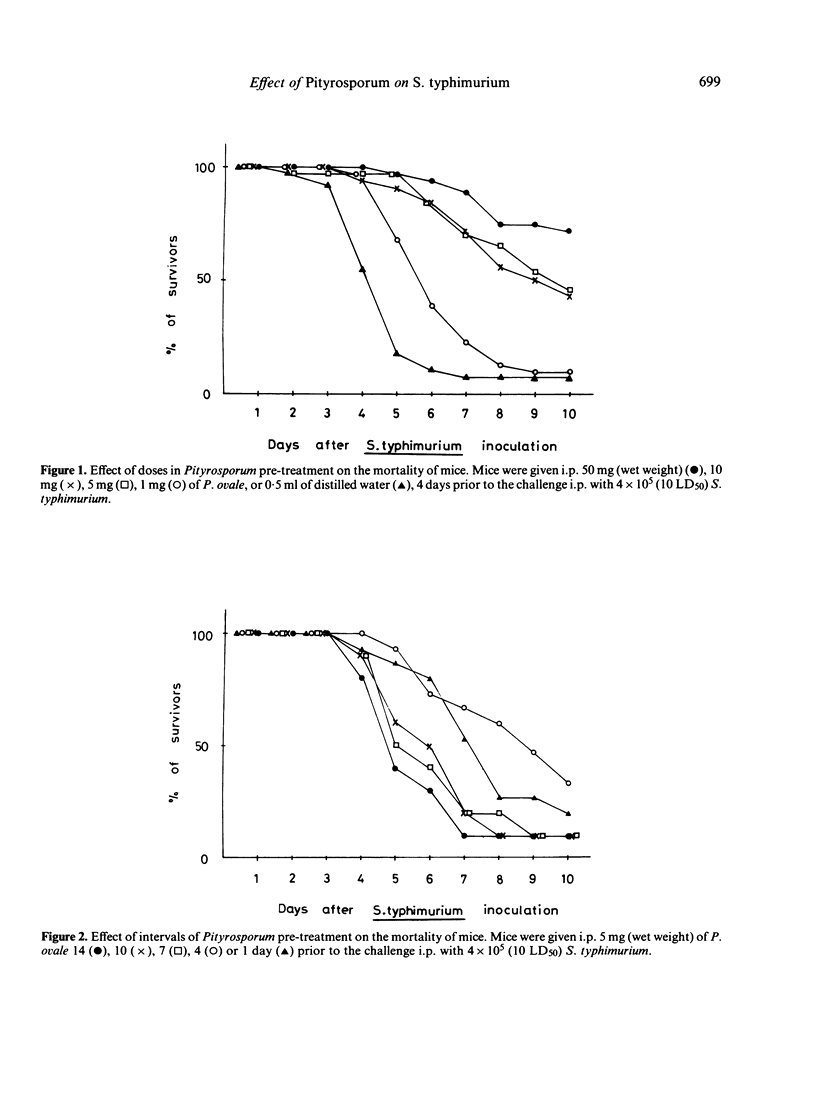
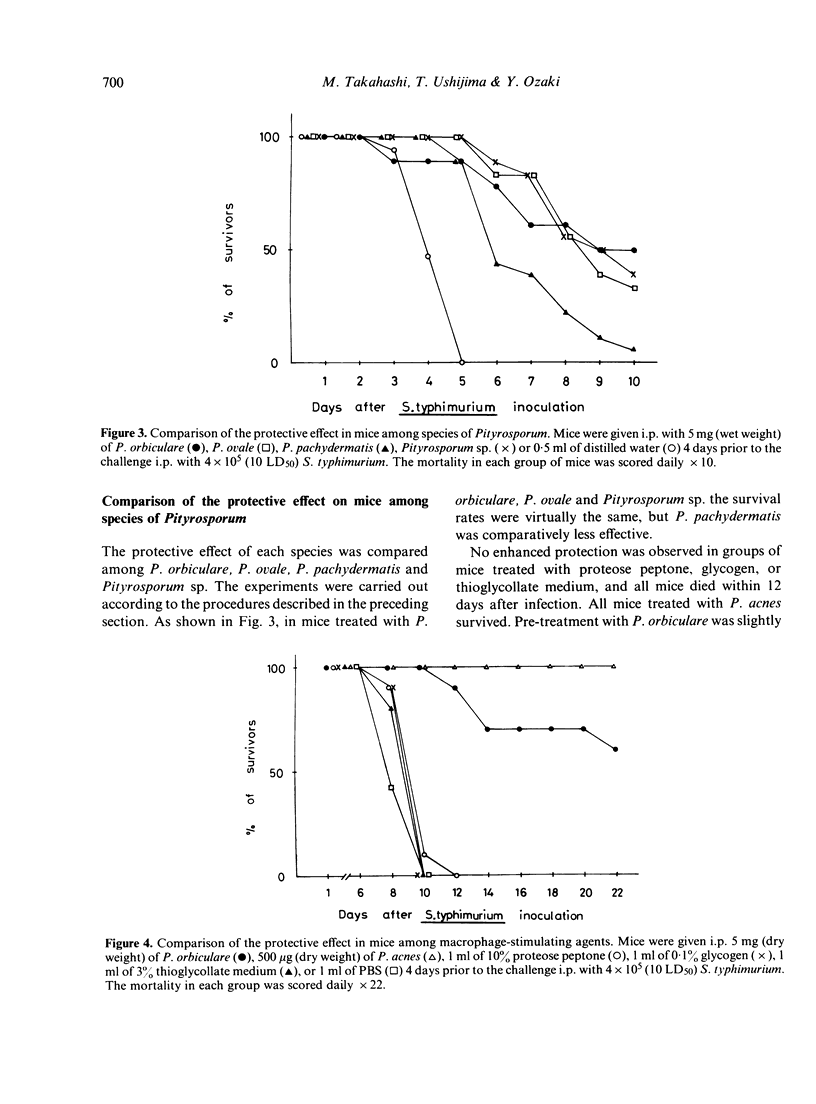
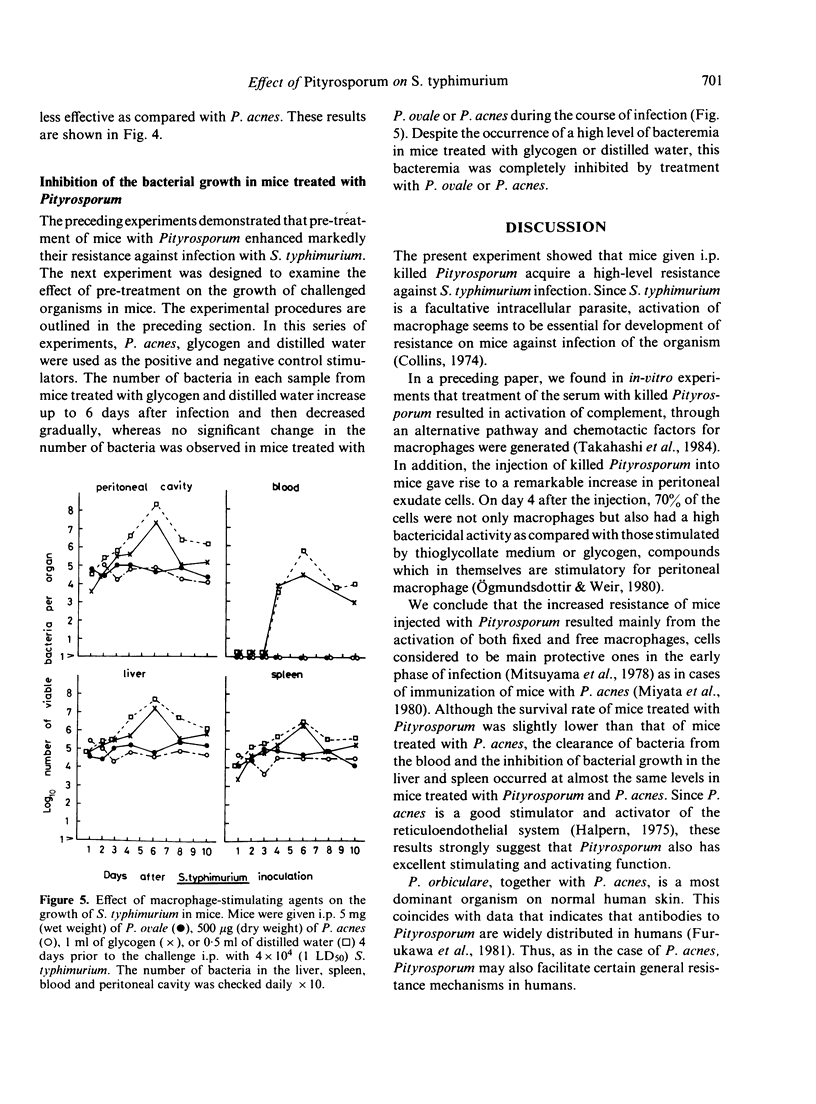
The effect of administration with Pityrosporum (P. orbiculare, P. ovale, P. pachydermatis and Pityrosporum sp.) on susceptibility of mice to Salmonella typhimurium infection was studied. Pretreatment of mice with 50 mg (wet weight) of killed Pityrosporum 4 days prior to the intraperitoneal (i.p.) challenge of 4 X 10(5) (10 LD50) S. typhimurium elicited resistance comparable to that induced by 500 micrograms (dry weight) of killed Propionibacterium acnes and over 30% of the infected mice survived. Among the species tested, P. pachydermatis was slightly less effective. The challenged organisms were not detected from the blood of mice treated with Pityrosporum but were present in the liver and spleen in approximately level amounts (10(4)-10(5)/organ) during the course of testing. These results suggest that the increased resistance in mice is the result of stimulation of the reticuloendothelial system by Pityrosporum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faergemann J. Experimental tinea versicolor in rabbits and humans with Pityrosporum orbiculare. J Invest Dermatol. 1979 Jun;72(6):326–329. doi: 10.1111/1523-1747.ep12531766. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A., Fischbach J., Bryant S. M., Kern E. R. Immunomodulation of host resistance to experimental viral infections in mice: effects of Corynebacterium acnes, Corynebacterium parvum, and Bacille calmette-guérin. J Infect Dis. 1977 May;135(5):763–770. doi: 10.1093/infdis/135.5.763. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Musselwhite M. S. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol. 1975 Sep;30(3):381–385. doi: 10.1128/am.30.3.381-395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marples R. R. Diphtheroids of normal human skin. Br J Dermatol. 1969;81(Suppl):47+–47+. doi: 10.1111/j.1365-2133.1969.tb12833.x. [DOI] [PubMed] [Google Scholar]
- McGinley K. J., Leyden J. J., Marples R. R., Kligman A. M. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975 Jun;64(6):401–405. doi: 10.1111/1523-1747.ep12512335. [DOI] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Ogmundsdóttir H. M., Weir D. M. Mechanisms of macrophage activation. Clin Exp Immunol. 1980 May;40(2):223–234. [PMC free article] [PubMed] [Google Scholar]


