Abstract
Covalent lipid attachments are essential co- and post-translational modifications for signalling proteins. Gαs, the α-subunit of the heterotrimeric G protein that activates adenylyl cyclase, is known to be palmitoylated at the third N-terminal amino acid, a cysteine. Palmitoylation is involved in anchoring Gαs to the membrane by increasing its intrinsic hydrophobicity. We identified by mass spectrometry a second, functionally even more important, covalent modification. It consists of another palmitoyl residue attached to the preceding glycine (Gly2). Palmitoylation at this position has profound consequences for levels of signal transduction. It sensitizes the cell up to 200-fold for adenylyl cyclase-stimulating agents. The inhibitory inputs mediated by Gαi are downregulated to <10%. Thereby, Gly2-palmitoylation of Gαs relieves cellular stimulation at the level of adenylyl cyclase whereas it renders the inhibitory modulation via Gαi more difficult.
Keywords: adenylyl cyclase/Gly2-palmitoylation/lipid modification/stimulatory G protein
Introduction
Heterotrimeric G proteins play a pivotal role in cellular signalling. They act as molecular switches that couple diverse receptors in the plasma membrane to a variety of intracellular effectors or signal generators. Efficiency of signalling depends on individual expression levels of the signalling partners and, even more importantly, their correct localization in the signalling compartment. Not until recently did it become evident that lipid modifications are an important determinant for localizing a protein into membranes and microdomains therein (Casey, 1995; Simons and Toomre, 2000).
G proteins are richly modified by lipids, which confer functional attributes such as protein–protein interactions (Linder et al., 1990; Casey, 1994; Tu et al., 1997; Chen and Manning, 2001) and a propensity for these otherwise soluble proteins to associate with membranes. The α-subunits of members of the Gi subfamily contain amide-linked myristate, and all Gα subunits (except Gαt) contain palmitate in thioester linkage on one or more nearby cysteine residues.
Myristoylation occurs during protein translation, and the saturated C14-lipid is permanently attached to the G protein on the N-terminal glycine residue (Gly2) that constitutes the N-terminus after removal of the initiating methionine Met1. Myristoylated G proteins adopt substantial functional features for cell signalling (Mumby et al., 1990), with targeting Gα to membranes being the most important.
In contrast, palmitoylation usually occurs at cysteine residues, is a dynamic protein modification (Cys-palmitoylation; Degtyarev et al., 1993; Mumby et al., 1994; Chen and Manning, 2000), and imparts pronounced hydrophobicity to the Gα subunit (Peitzsch and McLaughlin, 1993). Differences in opinion exist concerning the general functional consequences of G protein palmitoylation: be it membrane anchorage (Wedegaertner et al., 1993; Grassie et al., 1994) and/or specific interaction with regulators (Tu et al., 1997) and/or the selective targeting into membrane subdomains (Moffett et al., 2000; Waheed and Jones, 2002). For proteins that are acylated by both fatty acids, myristoylation usually precedes palmitoylation. So far, the functional consequences of myristoylation for the proteins role in signal transduction are thought to be dominant over potential effects of Cys-palmitoylation.
Gαs, discovered for its ability to stimulate production of the second messenger cAMP, is Cys3-palmitoylated like members of the Gαi subfamily, but it is not myristoylated, despite the presence of an N-terminal glycine residue. However, it was consistently observed that Gαs purified from sources such as mammalian liver or brain (we refer to it as ‘native Gαs’) has a higher apparent affinity for its effector adenylyl cyclase than does the mammalian protein that had been expressed in and purified from bacteria (we refer to it as ‘recombinant Gαs’). Previous experiments excluded the possibility that the Cys3-attached palmitoyl moiety of Gαs was responsible for this characteristic (Kleuss and Gilman, 1997). However, we reported the presence of an additional, at the time unidentified, lipophilic modification at or near the N-terminus of native Gαs. Here, we determined this modification to be a second palmitoyl residue that is linked to the G protein at the extreme N-terminal glycine by an unusual amide linkage. The functional consequences of this modification for the interaction of Gαs with adenylyl cyclase and plasma membrane localization are profound and are discussed in the context of signal transduction.
Results
Mass spectrometric analysis
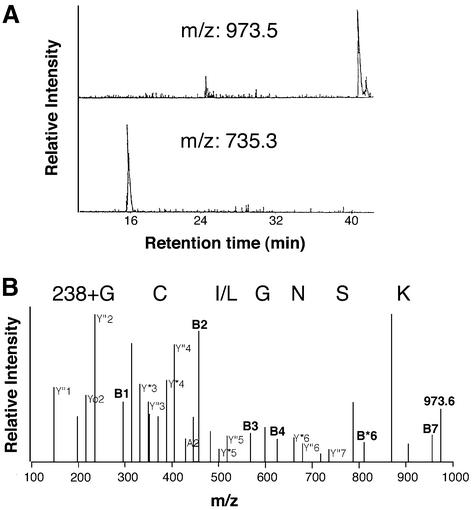
To identify the lipophilic modification at the N-terminus of Gαs, we analysed both the short and long splice variants of native Gαs and the recombinant protein by peptide mass fingerprinting. Analysis of mass spectra of recombinant Gαs with regard to the presence of the N-terminal sequence revealed a mass peak at m/z 735.3, corresponding to the unmodified fragment amino acid 2–8 (GCLGNSK) after trypsin digestion. The evidence for an unmodified N-terminus is based on the B- and Y-ions of the CID spectrum (retention time 12.5 min) of on-line liquid chromatography electrospray ionization tandem mass spectrometry (LC-MS/MS; Table I). Mass peaks corresponding to unmodified N-terminal sequences were not present when native, purified Gαs protein was analysed by matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) or LC-MS/MS. However, we found distinct mass peaks at m/z 973.5 in MALDI spectra of Lys-C and tryptic digests of native Gαs that were not present in recombinant Gαs spectra (Figure 1A). We concluded that the native protein carried an additional group at the N-terminus with a mass of 238, corresponding to the size of a palmitoyl moiety.
Table I. MS/MS fragment ions generated from the N-terminal tryptic peptide of recombinant Gαs [M + H]+ = 735.3.
| Sequence | Fragment ion | m/zcalc | m/zfound |
|---|---|---|---|
| GC | B2 | 218.1 | 218.3 |
| GCLG | B4 | 388.2 | 388.4 |
| K | Y”1 | 147.1 | 147.3 |
| SK | Y”2 | 234.1 | 234.2 |
| NSK | Y”3 | 348.2 | 348.3 |
| NSK | Y*3 | 331.2 | 331.2 |
| GNSK | Y*4 | 388.2 | 388.4 |
Based on the single-charge precursor ion the MS/MS spectrum showed N-terminal and C-terminal fragment ions. The common nomenclature for fragment ions was used, i.e. B for N-terminal, Y” for C-terminal ions. (*) indicates the neutral loss of 17 mass units. Cysteine was modified by carbamidomethylation.
Fig. 1. Mass spectrometric analysis of Gαs. Native or recombinant Gαs was in-gel digested and the resulting peptides analysed by MALDI-MS. Mass peaks with m/z 973.5 and 735.3 corresponded to the palmitoylated and non-palmitoylated N-terminal sequence G2CLGNSK of Gαs, respectively. (A) LC chromatogram recorded for the native protein at m/z 973.5 and the recombinant protein at m/z 735.3. (B) MS/MS spectrum of m/z = 973.58 of native Gαs. Relevant ions were labelled according to the accepted nomenclature and listed in Table II.
The site of modification was determined by LC-MS/MS as the N-terminal glycine (Figure 1B; Table II). Additional MS/MS experiments (data not shown) on the native Gαs N-terminal peptide prepared by tryptic in-gel digestion using 4-vinylpyridine instead of iodoacetamide for cysteine alkylation, and MALDI-MS of cyanogen bromide digested native Gαs confirmed these results.
Table II. MS/MS fragment ions generated from the N-terminal Lys-C peptide of native Gαs (m/z 973.58).
| Sequence | Fragment ion | m/zcalc | m/zfound |
|---|---|---|---|
| Palm-G | B1 | 296.3 | 296.1 |
| Palm-GC | B2 | 456.3 | 456.2 |
| Palm-GCL | B3 | 569.4 | 569.0 |
| Palm-GCLG | B4 | 626.4 | 626.3 |
| Palm-GCLGNS | B6* | 810.4 | 810.8 |
| K | Y1” | 147.1 | 146.8 |
| SK | Yo2 | 216.1 | 215.6 |
| SK | Y2” | 234.1 | 233.6 |
| NSK | Y3* | 331.2 | 330.8 |
| NSK | Y3” | 348.2 | 348.6 |
| GNSK | Y4* | 388.2 | 388.0 |
| GNSK | Y4” | 405.2 | 404.5 |
| LGNSK | Y5* | 501.3 | 501.4 |
| LGNSK | Y5” | 518.3 | 518.0 |
| CLGNSK | Y6* | 661.3 | 660.9 |
| CLGNSK | Y6” | 678.3 | 678.4 |
Based on the single-charge precursor ion the MS/MS spectrum showed N-terminal and C-terminal fragment ion series B1–B6 and Y1–Y6, respectively. (*) and (o) indicate the neutral loss of 17 and 18 mass units, respectively. Cysteine was modified by carbamidomethylation.
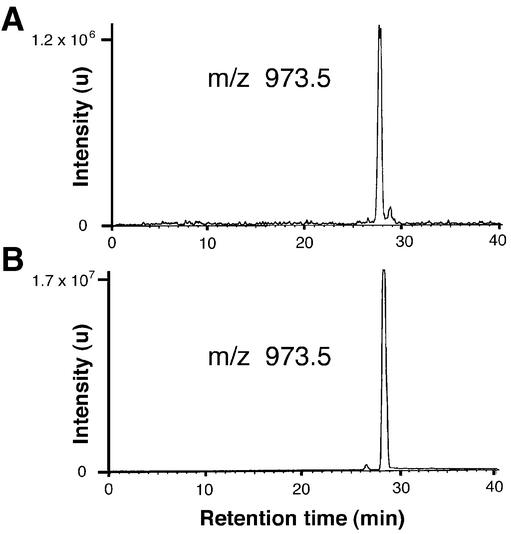
To prove the chemical nature of the attached lipid, we prepared N-terminal peptides by solid-phase synthesis and chemically attached a palmitoyl group at the N-terminal glycine. As shown in mass chromatograms (m/z 973.5), the N-terminus generated by in-gel digestion of native Gαs and the reference peptide, palm-GCLGNSK, exhibited identical masses and retention times in LC analysis (Figure 2). These data corroborated that the hitherto unknown modification is a palmitoyl residue that is attached to the N-terminal glycine of Gαs via a covalent amide bond.
Fig. 2. LC/MS analysis. Mass chromatogram (m/z 973.5) of the peptide mixture generated by in-gel digestion of native Gαs with Lys-C (A) and of the peptide palm-GCLGNSK prepared by solid-phase peptide synthesis (B). Samples were separated by HPLC and detected on-line by electrospray mass spectrometry. Indicated are signal intensities in arbitrary units (u).
In vitro palmitoylation
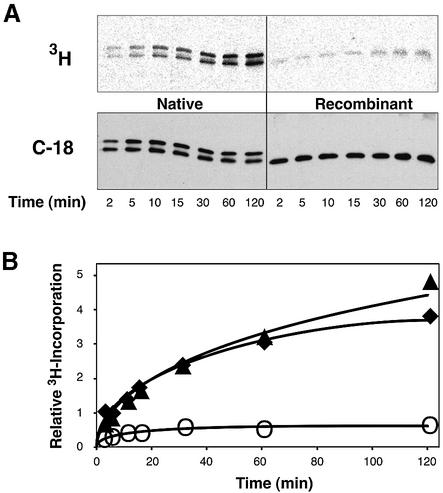
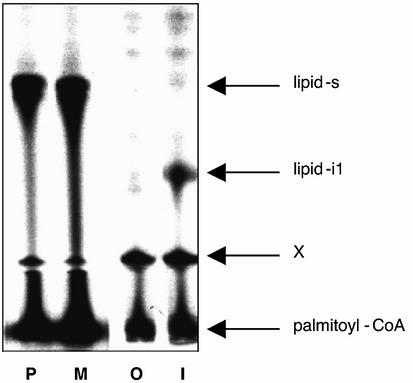
In a previous study, we showed that the lipophilic modification present on native Gαs (i.e. the Gly2-attached palmitoyl group) increased the apparent affinity of Gαs for adenylyl cyclase and the Gβγ complex (Kleuss and Gilman, 1997). Analogously, myristoylation at Gly2 enabled high affinity interactions of Gαi with the effector adenylyl cyclase (Taussig et al., 1993) and the Gβγ complex (Linder et al., 1991). Therefore, we speculated that other features may be shared by Gαi and Gαs, which are caused by the individual amide-linked acyl modification. Palmitoylation of Gαi and Gαo occurs spontaneously when the purified protein is incubated with palmitoyl– coenzyme A (Duncan and Gilman, 1996). These authors and others (Mollner et al., 1998) also showed that autopalmitoylation of Gα specifically occurred at Cys3. Incorporation of palmitate into Gαi is facilitated by the presence of amide-linked myristate at Gly2. Indeed, Gly2-palmitoylation of Gαs enhanced the spontaneous incorporation of radioactivity from tritiated palmitoyl–coenzyme A into the protein (Figure 3). This was corroborated by the in vitro palmitoylation of a synthetic peptide representing the seven N-terminal amino acids of Gαs under similar reaction conditions. The reaction was more efficient using a peptide that was already palmitoylated at Gly2 (Figure 4, lane P versus O).
Fig. 3. Autopalmitoylation of Gαs at Cys3. Gαs (2.5 µM) purified from recombinant bacteria (open circle) or native source (filled triangle for Gαs-short, filled diamond for Gαs-long) were incubated with 25 µM [9,10–3H]palmitoyl–coenzyme A (20 000 c.p.m./pmol) for the indicated times at 20°C. (A) Shown are 3H-images of reaction products after electrophoretic separation and transfer onto nitrocellulose membranes (upper panel), and immunostains with Gαs-specific antiserum C-18 (lower panel). (B) Relative 3H-incorporation into each protein was calculated from band intensities of the 3H-imaging normalized to the intensity of the corresponding immunostain. Recombinant Gαs was palmitoylated with a stoichiometry of ∼0.03 as determined by the filter binding assay described previously (Duncan and Gilman, 1996), implying >40% of native Gαs being palmitoylated after the 2 h incubation.
Fig. 4. Autopalmitoylation of peptides at Cys. Synthetic peptides representing the N-terminus of Gαs (P, M, O: GCLGNSK) or Gαi (I: GCTLSAEDK) were chemically modified at the N-terminal glycine by palmitate (P), myristate (M and I) or nothing (O). Peptides were subjected to autopalmitoylation and separated by thin-layer chromatography. Radioactivity was detected by fluorography. Shown is the fluorogram of a representative developed TLC plate. Arrows indicate the position of the bi-acylated N-terminal peptide of Gαs (lipid-s), bi-acylated N-terminal peptide of Gαi (lipid-i1), and the substrate palmitoyl–coenzyme A near to the origin. X denotes a non-identified spot that is already present in the substrate palmitoyl–coenzyme A and is located just above the preadsorbent/silica gel interface of the plate.
Stimulation of adenylyl cyclase
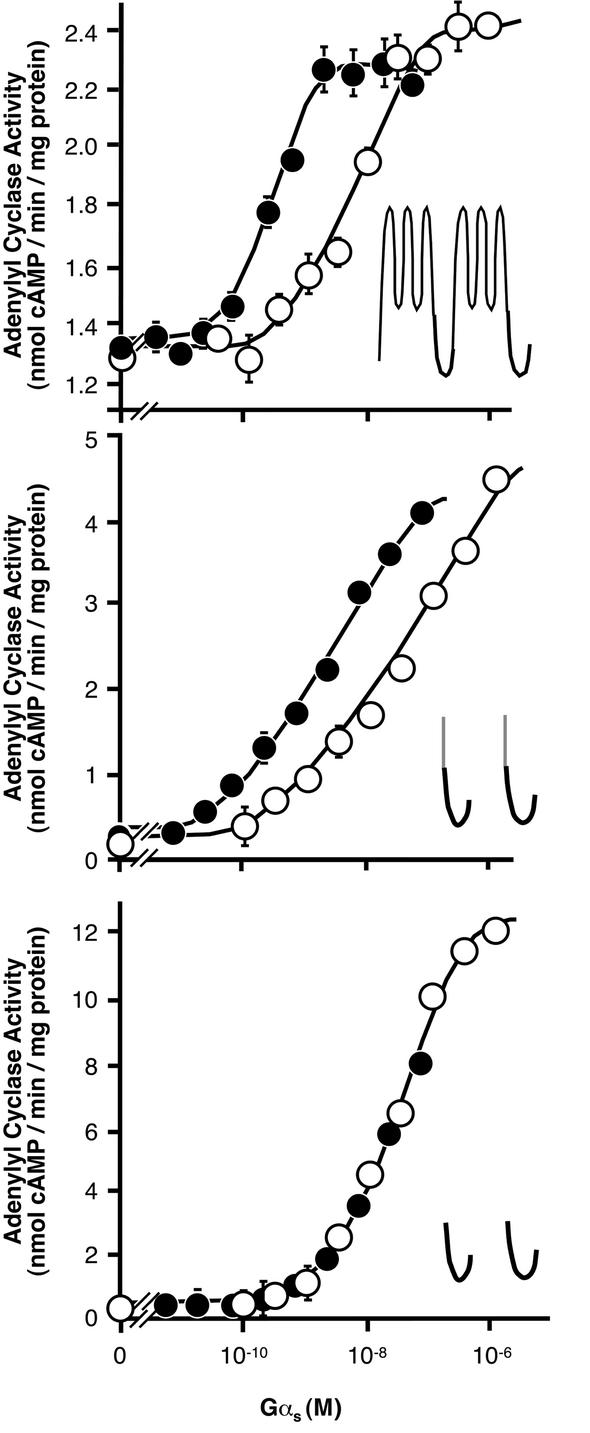
Native, Gly2-palmitoylated Gαs activated membrane-bound type I adenylyl cyclase with ∼60-fold higher apparent affinity than recombinant, unmodified Gαs (Figure 5, top). Similar observations were made on adenylyl cyclase type V, a mixture of several adenylyl cyclase subtypes (mainly type V and VI) or a bisected adenylyl cyclase (M1C1 + M2C2; Graziano et al., 1989; Kleuss and Gilman, 1997). According to the subtype of adenylyl cyclase used, the apparent Kd value of native Gαs was 0.1–0.3 nM, whereas recombinant Gαs stimulated the same adenylyl cyclases with an apparent Kd of 10–50 nM. For adenylyl cyclase type V, this resulted in an ∼200-fold higher affinity stimulation by Gly2-palmitoylated Gαs compared with recombinant Gαs. The effect of Gly2-palmitoylation on Gαs was not apparent with a soluble adenylyl cyclase (Figure 5, bottom). [Please note that ‘soluble adenylyl cyclase’ is an artificial construct of mammalian particulate adenylyl cyclase engineered to be soluble according to Tang et al. (Tang and Gilman, 1995) and does not refer to the mammalian soluble adenylyl cyclase expressed in testes.] In contrast, recombinant Gαs developed similar apparent affinities for all forms of adenylyl cyclase under the experimental conditions applied. There existed two major differences between the adenylyl cyclases used: (i) soluble adenylyl cyclase is devoid of amino acids representing the two hydrophobic clusters of mammalian particulate adenylyl cyclase; and (ii) soluble adenylyl cyclase is not localized at the membrane where particulate adenylyl cyclase naturally resides. To test whether the membrane localization of adenylyl cyclase was necessary for native Gαs to stimulate with high affinity, we anchored the soluble, catalytic parts of adenylyl cyclase into the plasma membrane by fusion to the heterologeous transmembrane span of the CD8-receptor (see Figure 5, centre). Concomitant expression of fusion constructs of both catalytic parts of adenylyl cyclase, CD8-C1 and CD8-C2, resulted in a particulate cAMP-generating enzyme. Gly2-palmitoylated Gαs stimulated this membrane-anchored adenylyl cyclase construct more efficiently than non-modified Gαs, indicating that high affinity stimulation was principally mediated by the membrane localization of the effector, not by topological or sequence-specific features of full-length adenylyl cyclase.
Fig. 5. Stimulation of adenylyl cyclase constructs by Gαs. Membranes (2 µg protein) containing particulate adenylyl cyclase or soluble protein (20 µg, partially purified by Ni2+–NTA agarose) were used. Increasing amounts of GTPγS-activated Gαs purified from bovine brain (native; closed circle) or bacterially expressed protein (recombinant; open circles) were added. Shown are adenylyl cyclase activities of full-length particulate adenylyl cyclase type I (top), soluble adenylyl cyclase halves anchored to the plasma membrane by fusion to the transmembrane span of the CD8-receptor (CD8-C1 + CD8-C2; center), and soluble adenylyl cyclase (bottom). The cartoons depict the putative topology of expressed adenylyl cyclase constructs; the CD8-span of adenylyl cyclase fusion construct is coloured grey. Values are representative of one experiment out of three similar assays performed in duplicates, SD is indicated.
Inhibition of adenylyl cyclase
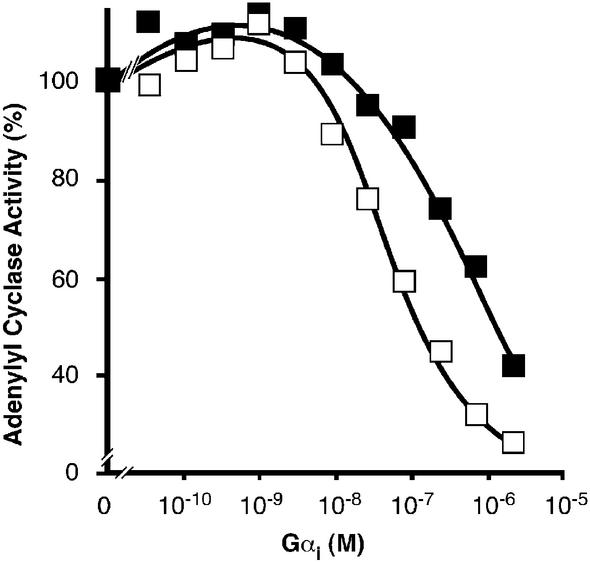
The increased affinity of Gly2-palmitoylated Gαs for particulate adenylyl cyclase not only enhanced the ability of Gαs to stimulate adenylyl cyclase, but also considerably attenuated the efficiency of Gαi to inhibit the same effector. Gαi reconstitution showed high affinity interaction and regulation of adenylyl cyclase type V and VI and, to a lesser extent, type I, depending on the status of Gαi lipid modification. Myristoylated Gαi inhibited adenylyl cyclase with an IC50 of ∼0.1 µM (Taussig et al., 1993), whereas non-myristoylated Gαi was inactive (Linder et al., 1990). The presence of the palmitoyl modification at Cys3 in addition to the Gly2-myristoylation did not affect these regulatory features of Gαi (C.Kleuss, unpublished results). The inhibitory action of myristoylated Gαi is only evident if adenylyl cyclase had been pre-stimulated, either by forskolin or naturally by Gαs. If recombinant Gαs was used for pre-stimulation, myristoylated Gαi efficiently inhibited adenylyl cyclase type V (Figure 6). If adenylyl cyclase had been pre-stimulated by native Gαs, myristoylated Gαi was a less-efficient inhibitor of cAMP-generating activity with an IC50 of 1 µM.
Fig. 6. Inhibition of adenylyl cyclase. Recombinant adenylyl cyclase type V in insect cell membranes (10 µg protein) was reconstituted with GTPγS-activated myristoylated Gαi to give the indicated final concentrations in the presence of 0.1 nM GTPγS-bound native Gαs (filled squares) or 20 nM GTPγS-bound recombinant Gαs (open squares). Please note, we determined the inhibitory action of myristoylated Gαi at comparable levels of adenylyl cyclase pre-stimulated activity by using only 1/200 the amount of Gly2-palmitoylated than non-palmitoylated Gαs. Pre-stimulated adenylyl cyclase activity in the absence of Gαi (100% activity) was 3.8 nmol/min/mg protein for recombinant Gαs and 4.2 nmol/min/mg protein for native Gαs. Values are representative of one experiment out of three similar assays, mean SEM of duplicates was below 1%.
Discussion
Native Gαs is modified at the N-terminal glycine (Gly2 after removal of the starter methionine) by a palmitoyl residue, as shown by mass spectrometric analysis. The Gly2-palmitoylation was determined on both native Gαs splice variants, and is presumed to be a permanent modification due to the chemical stability of the amide-linkage as evident by its stability during the lengthy purification procedure of native Gαs.
Gly2-palmitoylation and Cys3-palmitoylation
Our mass spectrometric data revealed only one palmitoyl group at the N-terminus of Gαs (at Gly2). Cys3-palmitoylation is reversible under mild experimental conditions, while Gly2-palmitoylation is permanent and stable. Therefore, native Gαs most probably was already devoid of Cys3-attached palmitate in the end of our purification procedure. Nevertheless, prior to mass spectrometric analysis, we modified all cysteines present in the protein to remove disulfide bonds so that any residual S-palmitoyl modification (at Cys3) would also have been removed from Gαs. For the biochemical characterization, the Cys3-palmitoylation status of Gαs was irrelevant, as previously described (Kleuss and Gilman, 1997). Under cellular conditions, Gαs most probably is temporarily palmitoylated on both residues, Gly2 and Cys3. The two neighbouring palmitoyl groups in Gαs are not equivalent, in contrast to other dually palmitoylated signalling proteins known to date. The newly described palmitoyl group on Gly2 is attached via an amide linkage, while the palmitoyl group at Cys3 is attached via a thioester bond (Linder et al., 1993). So far, palmitoylation via an N-terminal glycine was unknown to occur on heterotrimeric G proteins. Furthermore, palmitoylation via an amide bond (N-terminal cysteine or internal lysine) seems to be rare. N-palmitoylation has been reported for the vertebrate Sonic hedgehog protein (Pepinsky et al., 1998) and for the adenylyl cyclase of Bordetella pertussis (Hackett et al., 1994). Palmitoylation of Sonic hedgehog, which occurs via an amide bond at the N-terminal cysteine, is required for its embryonic patterning activities. The adenylyl cyclase of B.pertussis, consisting of 1706 amino acids, is internally palmitoylated at Lys983. Toxicity of this adenylyl cyclase (i.e. the cell-invasive activity) is directly dependent on the palmitoylation at Lys983. This exclusive group of molecules that gain their signalling abilities by N-palmitoylation is now extended to Gαs. The distinct chemical nature of the two acyl anchors of Gαs implies different mechanisms of palmitoylation and potentially indicates divergent functional consequences for the protein. In particular, the functional consequences of Gly2-palmitoylation of Gαs resemble those of myristoylation of Gαi (at Gly2; both proteins can be additionally acylated by palmitate at their Cys3) rather than the doubly palmitoylated Gαq (palmitoylation at Cys9 and Cys10).
Palmitoyl transfer reaction
The existence of a palmitoylated Gly2 in Gαs adds a new dimension to the discussion about palmitoyltransferases. Although palmitoylation was first described over thirty years ago, the molecular mechanism of palmitate addition has been a matter of controversy. An activity capable of palmitoylating Gα has been detected in mammalian plasma membranes (Dunphy et al., 1996). Unfortunately, the proteins responsible for this activity defied purifica tion till now (Berthiaume and Resh, 1995; Dunphy et al., 2001). On the other hand, myristoylated Gα subunits can be palmitoylated at the same sites that become palmitoylated in vivo in the absence of a specific acyltransferase using palmitoyl–coenzyme A as the acyl donor (Duncan and Gilman, 1996), raising the question of the requirement for an exogenous enzyme. Skinny hedgehog/sightless was identified as a palmitoyltransferase for Drosophila hedgehog (Chamoun et al., 2001). Hedgehog is palmitoylated through an atypical cysteine amide linkage at the N-terminus. However, it seems unlikely that skinny hedgehog can palmitoylate Gαs at Gly2, as both proteins are not homologous in their N-termini and, moreover, the target amino acid in hedgehog is a cysteine residue.
Recently, a palmitoyltransferase that acylates through a conventional thioester linkage, was identified in yeast (Lobo et al., 2002). Erf2p/Erf4p can directly mediate palmitate transfer to yeast Ras using palmitoyl– coenzyme A as a donor. It remains to be shown that mammalian homologues of Erf2p/Erf4p act as palmitoyltransferases on mammalian Ras and/or Gα proteins (Cys3). Nevertheless, a putative Erf2p/Erf4p homologue is most likely not the catalyst for the Gly2 palmitoylation of Gαs. However, it is conceivable that a transacylation reaction from Cys3 onto Gly2 takes place. A similar reaction mechanism was already described for Sonic hedgehog N-terminal palmitoylation where palmitate is transferred from the thioester-bonded cysteine onto the amino group of the same cysteine after removal of the N-terminal signal peptide. The palmitate-transacylation reaction is compatible with preliminary data on Cys3 mutants that prevent Gly2-palmitoylation (data not shown).
Gly2-palmitoylation impacts Cys3-palmitoylation of Gαs
In vivo, the exchange of palmitate at Cys3 in Gαs and Gαi is enhanced upon stimulation of the coupling receptor through the combined processes of depalmitoylation and palmitoylation (Mumby et al., 1994; Chen and Manning, 2000). Wang et al. (1999) described that, beyond membrane anchorage, myristoylation of Gαo (at Gly2) is important for the subsequent Cys3-palmitoylation. Similarly, for Gαs we show here that Gly2-palmitoylation facilitated the incorporation of the additional, reversibly attached palmitoyl residue at Cys3 thereby enabling Gαs to switch between a lower (Gly2-palmitoylated Gαs) and higher hydrophobicity (Gly2- plus Cys3-palmitoylated protein). We observed increased Cys3-autopalmitoylation upon Gly2-palmitoylation in both, the short and long, splice variants of native Gαs. Autoacylation was also enhanced in short synthetic peptides used in model reactions to represent the N-termini of Gαs and Gαi. Apparently, the exact chemical nature of the lipid attached to Gly2 was less important. A synthetic peptide representing the N-terminus of Gαs modified with myristate at the N-terminal glycine residue, i.e. a non-physiological modification for Gαs, incorporated radioactivity as well as the palmitoylated peptide (see Figure 4, lane M). The underlying mechanism may be the preferred distribution of native Gαs or the corresponding Gly-acylated peptide into detergent micelles that harbour the substrate, palmitoyl– coenzyme A, thereby increasing the local concentration of the reaction partners and the mass-driven reaction.
Gly2-palmitoylation of Gαs impacts regulation of adenylyl cyclase
A similar distribution phenomenon may contribute to the mechanism that renders Gly2-palmitoylated Gαs more efficient to stimulate particulate adenylyl cyclase: Gly2-palmitoylation directs Gαs into the plasma membrane to locally increase the protein concentration required for an efficient stimulatory coupling to the membrane-bound adenylyl cyclase. It is still unknown whether Gly2-palmitoylation of Gαs also enables a lipid–protein interaction with specific transmembrane regions of adenylyl cyclase. Such a lipid–protein contact would require a second binding site of Gαs on adenylyl cyclase, i.e. an additional one to the protein–protein interaction domains shown in the crystal structure of adenylyl cyclase complexed with recombinant Gαs (Sunahara et al., 1997). This possibility seems, however, to be somewhat remote because the mere attachment of the adenylyl cyclase catalyst via the CD8 anchor already was sufficient to observe the functional consequences of Gly2-palmitoylation at Gαs on adenylyl cyclase activity. However, we are aware of the different shaped concentration–activity curves of full-length adenylyl cyclase and the membrane-anchored constructs indicating some, although not a major, function of transmembrane spans of adenylyl cyclase in Gαs activation. We also observed subtle differences of native Gαs affinity for particulate adenylyl cyclase chimeras consisting of transmembrane spans derived from different adenylyl cyclase subtypes (mixed from adenylyl cyclase type I and II; data not shown). Taken together, we cannot exclude a specific lipid contact with adenylyl cyclase transmembrane domains formed by both clusters in an isoform-specific manner.
A lipophilic binding site in adenylyl cyclase transmembrane domains could also explain the different efficiencies of myristoylated Gαi to inhibit adenylyl cyclase type V according to the type of Gαs protein used for pre-stimulation. From three-dimensional structure and mutational analysis, it is evident that Gαs and Gαi interact with diagonally separated sites of distinct cytoplasmic halves of adenylyl cyclase. Therefore, the likely mechanism exerted by both Gα subunits is an allosteric adenylyl cyclase regulation rather than a direct competition at those effector domains (Taussig et al., 1993). Surprisingly, myristoylated Gαi was a less efficient inhibitor of adenylyl cyclase if pre-stimulation was performed with 0.1 nM Gly2-palmitoylated Gαs compared with 20 nM non-palmitoylated Gαs. Therefore, an additional contact between the transmembrane domains of adenylyl cyclase and the acyl modification of one or the other Gα subunit seemed conceivable. In such a scenario, the different acyl chains attached to Gα might compete for a similar lipophilic binding site in adenylyl cyclase transmembrane domains, thereby superimposing the allosteric regulation mediated by adenylyl cyclase cytosolic domains and the membrane-delimited competition.
Irrespectively of the underlying mechanism, Gly2-palmitoylation of Gαs has profound functional consequences for Gs-mediated signal transduction at the level of adenylyl cyclase regulation. Gly2-palmitoylated Gαs (in contrast to non-palmitoylated or Cys3-palmitoylated Gαs) renders the cell more receptive for stimulatory inputs and less sensitive for inhibitory stimuli that are transmitted by G protein α-subunits. This process adds a new layer of regulatory possibilities to this exceedingly important signal transduction system.
Materials and methods
In-gel digestion
Native and recombinant Gαs were subjected to polyacrylamide gel electrophoresis. After visualization and destaining, Gαs spots were excised, washed with 50% (v/v) acetonitrile in 25 mM ammonium bicarbonate, shrunk by dehydration in acetonitrile and dried in a vacuum centrifuge. Disulfide bonds were reduced by incubation in 30 µl of 10 mM DTT/100 mM ammonium bicarbonate in water (45 min at 55°C). Alkylation was performed by replacing the DTT solution with 55 mM iodoacetamide or 90 mM 4-vinylpyridine in 25 mM ammonium bicarbonate. After 20 min incubation at 25°C in the dark, the gel pieces were washed with 50–100 µl of 50% (v/v) acetonitrile in 25 mM ammonium bicarbonate, shrunk by dehydration in acetonitrile and dried in a vacuum centrifuge. The gel pieces were re-swollen in 10 µl of 5 mM ammonium bicarbonate, containing 500 ng endoproteinase Lys-C or trypsin (sequencing grade; Roche Diagnostics, Mannheim, Germany). After 15 min, 5 µl of 5 mM ammonium bicarbonate was added to keep the gel pieces wet during cleavage (37°C, overnight). For extraction, 15 µl of 0.5% (v/v) trifluoroacetic acid (TFA) in acetonitrile was added, followed by sonication for 5 min.
MALDI-MS
Measurements were performed on a Voyager-DE STR BioSpectrometry Workstation MALDI-TOF mass spectrometer (Perseptive Biosystems, Inc., Framingham, MA). Briefly, samples were prepared with α-cyano-4-hydroxycinnamic acid matrix and measured in the reflection mode at an acceleration voltage of 20 kV and a delay of 200 ns. Peptides from the tryptic and Lys-C digested proteins covered 71% (281 out of 394 amino acids) of the protein (SwissProt accession No. P04896).
LC-MS/MS
For on-line LC-MS measurements, 10 µl of the peptide mixture from in-gel digestion was dried under vacuum, redissolved in 5 µl of 20% (v/v) acetonitrile/0.1% (v/v) TFA in water, and subjected to reversed-phase HPLC separation on a C18 column (Vydac 218TP5115, 150 × 1 mm i.d., 5 µm, Promochem, Wesel, Germany). Elution was performed at a flow rate of 30 µl/min generated by a model 140B Applied Biosystems solvent delivery system. Mobile phase A was 0.06 % TFA in water and B was 0.05% TFA in acetonitrile–water (8:2, v/v). Runs were performed using a linear gradient of 25–95% B and 50–95% B in 40 min for recombinant and native Gαs, respectively. Mass spectrometry was performed on-line on a triple quadrupole instrument (TSQ 700, Finnigan MAT, Bremen, Germany) equipped with the standard electrospray ion source operating in the positive mode with a capillary temperature of 200°C and a voltage of 4.5 kV. Nitrogen was introduced as sheath gas at a pressure of 3.4 bar and auxiliary gas (flow 960 ml/min). A sheath liquid of isopropanol– propionic acid (25:75, v/v) was applied at a flow rate of 4 µl/min. Total ion chromatograms and mass spectra (scan range 300–1600 m/z) were acquired. Fragment ion spectra were generated by collision-induced dissociation (CID) using argon as collision gas (3–4 mTorr) and collision energies ranging from 20 to 40 eV.
Peptide synthesis
Synthetic peptides representing the N-terminus of Gαs (GCLGNSK) were synthesized by solid-phase methods using Fmoc (Nα-9-fluorenylmethoxycarbonyl) chemistry in the continuous flow mode (Beyermann et al., 1996). The N-terminal glycine was acylated with palmitic acid or myristic acid using the amino acid coupling chemistry. After cleavage from the resin support, cysteine alkylation was performed by 55 mM iodoacetamide in 100 mM ammonium bicarbonate. Peptides were purified by preparative high-performance liquid chromatography to give final products of >95% purity.
Autopalmitoylation
Autopalmitoylation was performed according to Duncan and Gilman (1996). Recombinant Gαs protein was used as the C-terminal H6-tagged protein as described previously (Kleuss and Gilman, 1997). Native Gαs was purified from bovine brain. Due to the lengthy purification procedure in the presence of 1–3 mM DTT, the native Gly2-palmitoylated Gαs used throughout this study was not supposed to contain Cys3-attached palmitate. Briefly, 2.5 µM Gαs was incubated with 25 µM [9,10–3H]palmitoyl–coenzyme A (20 000 c.p.m./pmol) for 90 min if not otherwise indicated. Peptides were solubilized in DMSO and autopalmitoylated under similar conditions [final concentration 1% (w/v) DMSO]. Proteins were separated by polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Dried membranes were exposed to 3H-imaging screens before proteins were immunostained with Gαs-specific antiserum C-18. Autoacylated peptides were applied to thin layer chromatography on discontinuous silica plates (Whatman LK6D Silica Gel, 60 Å) and developed in n-butanol: pyridine:acetic acid:water (60:30:5:15) for 3 h. Radioactivity was detected by fluorography.
Adenylyl cyclase
Adenylyl cyclases and mutants thereof were expressed in Sf9 cells using recombinant baculoviruses (Weitmann et al., 2001). Viruses encoding adenylyl cyclase type I and V were kindly provided by the laboratory of A.G.Gilman. Generation of viruses encoding bisected adenylyl cyclases type I and II was described previously (Weitmann et al., 1999), as well as generation of viruses encoding the soluble His6-tagged adenylyl cyclase (Weitmann et al., 2001). Fusion constructs of CD8 (coding for amino acids 1–217 of the CD8 receptor; GI number 22902133) with sequences coding for domain C1 of adenylyl cyclase type I and domain C2 of adenylyl cyclase type II, were generated by PCR using SalI recognition site as junction. The extracellular portion of the CD8 receptor was proposed to dimerize. Consequently, dimerization of the membrane anchored catalytic parts of adenylyl cyclase was alleviated. All adenylyl cyclases were expressed for 48–55 h in insect cells; cells were harvested and plasma membranes or cytosol were prepared by nitrogen cavitation. Membranes were washed and flash frozen as well as cytosolic fractions. Adenylyl cyclase activity was measured for 10 min at 30°C in the presence of 2 µM forskolin and/or Gαs as indicated (Kleuss and Gilman, 1997).
Miscellaneous
Immunoblotting was performed with commercially available antibodies (Santa Cruz). Labelled samples on nitrocellulose membranes or silica plates were visualized by 3H-imaging system (BAS-1500, Fuji Film) or exposure to Biomax MS films (Kodak); quantification was performed with IPLab Gel (Signal Analytics, Vienna, VA).
Acknowledgments
Acknowledgements
We gratefully acknowledge A.G.Gilman and members of his research group for encouragement and advice during the initiation of this project. In addition, we thank C.Belanger for advice in peptide labelling, M.Beyermann for chemical peptide synthesis and N.Würsig for excellent technical help. We appreciate the permanent and constructive support by M.E.Linder, S.M.Mumby and H.Schmidt. The work was supported by DFG (Kl 773/4; SFB 366/A14 to C.K.).
References
- Berthiaume L. and Resh,M.D. (1995) Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J. Biol. Chem., 270, 22399–22405. [DOI] [PubMed] [Google Scholar]
- Beyermann M., Fechner,K., Furkert,J., Krause,E. and Bienert,M. (1996) A single-point slight alteration set as a tool for structure-activity relationship studies of ovine corticotropin releasing factor. J. Med. Chem., 39, 3324–3330. [DOI] [PubMed] [Google Scholar]
- Casey P.J. (1994) Lipid modifications of G proteins. Curr. Opin. Cell Biol., 6, 219–225. [DOI] [PubMed] [Google Scholar]
- Casey P.J. (1995) Protein lipidation in cell signaling. Science, 268, 221–225. [DOI] [PubMed] [Google Scholar]
- Chamoun Z., Mann,R.K., Nellen,D., von Kessler,D.P., Bellotto,M., Beachy,P.A. and Basler,K. (2001) Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science, 293, 2080–2084. [DOI] [PubMed] [Google Scholar]
- Chen C.A. and Manning,D.R. (2000) Regulation of Gαi palmitoylation by activation of the 5-HT1A receptor. J. Biol. Chem., 275, 23516–23522. [DOI] [PubMed] [Google Scholar]
- Chen C.A. and Manning,D.R. (2001) Regulation of G proteins by covalent modification. Oncogene, 20, 1643–1652. [DOI] [PubMed] [Google Scholar]
- Degtyarev M.Y., Spiegel,A.M. and Jones,T.L. (1993) Increased palmitoylation of the Gs protein α subunit after activation by the β-adrenergic receptor or cholera toxin. J. Biol. Chem., 268, 23769–23772. [PubMed] [Google Scholar]
- Duncan J.A. and Gilman,A.G. (1996) Autoacylation of G protein α subunits. J. Biol. Chem., 271, 23594–23600. [DOI] [PubMed] [Google Scholar]
- Dunphy J.T., Greentree,W.K., Manahan,C.L. and Linder,M.E. (1996) G-protein palmitoyltransferase activity is enriched in plasma membranes. J. Biol. Chem., 271, 7154–9. [DOI] [PubMed] [Google Scholar]
- Dunphy J.T., Greentree,W.K. and Linder,M.E. (2001) Enrichment of G-protein palmitoyltransferase activity in low density membranes: in vitro reconstitution of Gαi to these domains requires palmitoyltransferase activity. J. Biol. Chem., 276, 43300–43304. [DOI] [PubMed] [Google Scholar]
- Grassie M.A., McCallum,J.F., Guzzi,F., Magee,A.I., Milligan,G. and Parenti,M. (1994) The palmitoylation status of the G-protein Go1α regulates its activity of interaction with the plasma membrane. Biochem. J., 302, 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M.P., Freissmuth,M. and Gilman,A.G. (1989) Expression of Gsα in Escherichia coli. Purification and properties of two forms of the protein. J. Biol. Chem., 264, 409–418. [PubMed] [Google Scholar]
- Hackett M., Guo,L., Shabanowitz,J., Hunt,D.F. and Hewlett,E.L. (1994) Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science, 266, 433–435. [DOI] [PubMed] [Google Scholar]
- Kleuss C. and Gilman,A.G. (1997) Gsα contains an unidentified covalent modification that increases its affinity for adenylyl cyclase. Proc. Natl Acad. Sci. USA, 94, 6116–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder M.E., Ewald,D.A., Miller,R.J. and Gilman,A.G. (1990) Purification and characterization of Goα and three types of Giα after expression in Escherichia coli. J. Biol. Chem., 265, 8243–8251. [PubMed] [Google Scholar]
- Linder M.E., Pang,I.H., Duronio,R.J., Gordon,J.I., Sternweis,P.C. and Gilman,A.G. (1991) Lipid modifications of G protein subunits. Myristoylation of Goα increases its affinity for βγ. J. Biol. Chem., 266, 4654–4659. [PubMed] [Google Scholar]
- Linder M.E., Middleton,P., Hepler,J.R., Taussig,R., Gilman,A.G. and Mumby,S.M. (1993) Lipid modifications of G proteins: α subunits are palmitoylated. Proc. Natl Acad. Sci. USA, 90, 3675–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S., Greentree,W.K., Linder,M.E. and Deschenes,R.J. (2002) Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem., 277, 41268–41273. [DOI] [PubMed] [Google Scholar]
- Moffett S., Brown,D.A. and Linder,M.E. (2000) Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem., 275, 2191–2198. [DOI] [PubMed] [Google Scholar]
- Mollner S., Ferreira,P., Beck,K., Pfeuffer,T. (1998) Nonenzymatic palmitoylation at Cys3 causes extra-activation of the α-subunit of the stimulatory GTP-binding protein Gs. Eur. J. Biochem. 257, 236–241. [DOI] [PubMed] [Google Scholar]
- Mumby S.M., Heukeroth,R.O., Gordon,J.I. and Gilman,A.G. (1990) G-protein α-subunit expression, myristoylation, and membrane association in COS cells. Proc. Natl Acad. Sci. USA, 87, 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S.M., Kleuss,C. and Gilman,A.G. (1994) Receptor regulation of G-protein palmitoylation. Proc. Natl Acad. Sci. USA, 91, 2800–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitzsch R.M. and McLaughlin,S. (1993) Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry, 32, 10436–10443. [DOI] [PubMed] [Google Scholar]
- Pepinsky R.B. (1998) Identification of a palmitic acid-modified form of human Sonic hedgehog. J. Biol. Chem., 273, 14037–14045. [DOI] [PubMed] [Google Scholar]
- Simons K. and Toomre,D. (2000) Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol., 1, 31–39. [DOI] [PubMed] [Google Scholar]
- Sunahara R.K., Tesmer,J.J., Gilman,A.G. and Sprang,S.R. (1997) Crystal structure of the adenylyl cyclase activator Gsα. Science, 278, 1943–1947. [DOI] [PubMed] [Google Scholar]
- Tang W.J. and Gilman,A.G. (1995) Construction of a soluble adenylyl cyclase activated by Gsα and forskolin. Science, 268, 1769–1772. [DOI] [PubMed] [Google Scholar]
- Taussig R., Iniguez-Lluhi,J.A. and Gilman,A.G. (1993) Inhibition of adenylyl cyclase by Giα. Science, 261, 218–221. [DOI] [PubMed] [Google Scholar]
- Tu Y., Wang,J. and Ross,E.M. (1997) Inhibition of brain Gz GAP and other RGS proteins by palmitoylation of G protein α subunits. Science, 278, 1132–1135. [DOI] [PubMed] [Google Scholar]
- Waheed A.A. and Jones,Z.T.L. (2002) Hsp90 interactions and acylation target the G-protein Gα12, but not Gα13 to lipid rafts. J. Biol. Chem., 277, 32409–32412. [DOI] [PubMed] [Google Scholar]
- Wang Y., Windh,R.T., Chen,C.A. and Manning,D.R. (1999) N-myristoylation and βγ play roles beyond anchorage in the palmitoylation of the G protein αo subunit. J. Biol. Chem., 274, 37435–37442. [DOI] [PubMed] [Google Scholar]
- Wedegaertner P.B., Chu,D.H., Wilson,P.T., Levis,M.J. and Bourne,H.R. (1993) Palmitoylation is required for signaling functions and membrane attachment of Gqα and Gsα. J. Biol. Chem., 268, 25001–25008. [PubMed] [Google Scholar]
- Weitmann S., Würsig,N., Navarro,J.M. and Kleuss,C. (1999) A functional chimera of mammalian guanylyl and adenylyl cyclases. Biochemistry, 38, 3409–3413. [DOI] [PubMed] [Google Scholar]
- Weitmann S., Schultz,G. and Kleuss,C. (2001) Adenylyl cyclase type II domains involved in Gβγ stimulation. Biochemistry, 40, 10853–10858. [DOI] [PubMed] [Google Scholar]