Abstract
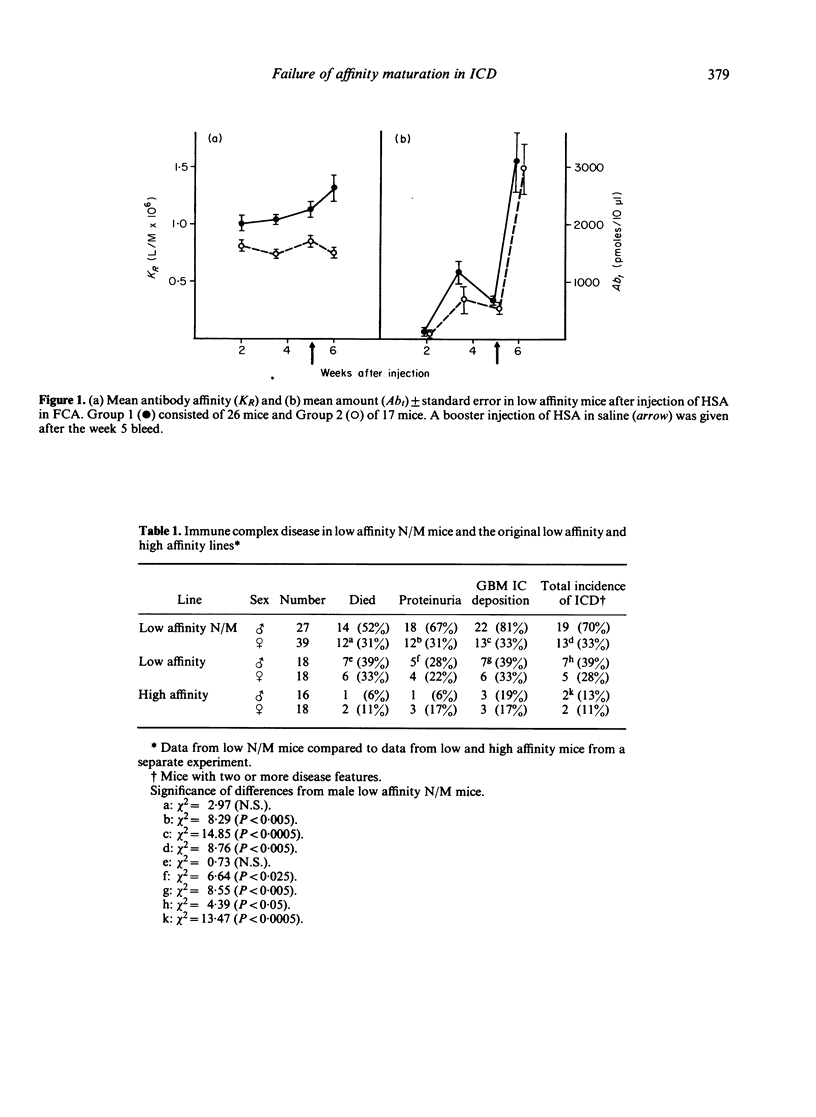
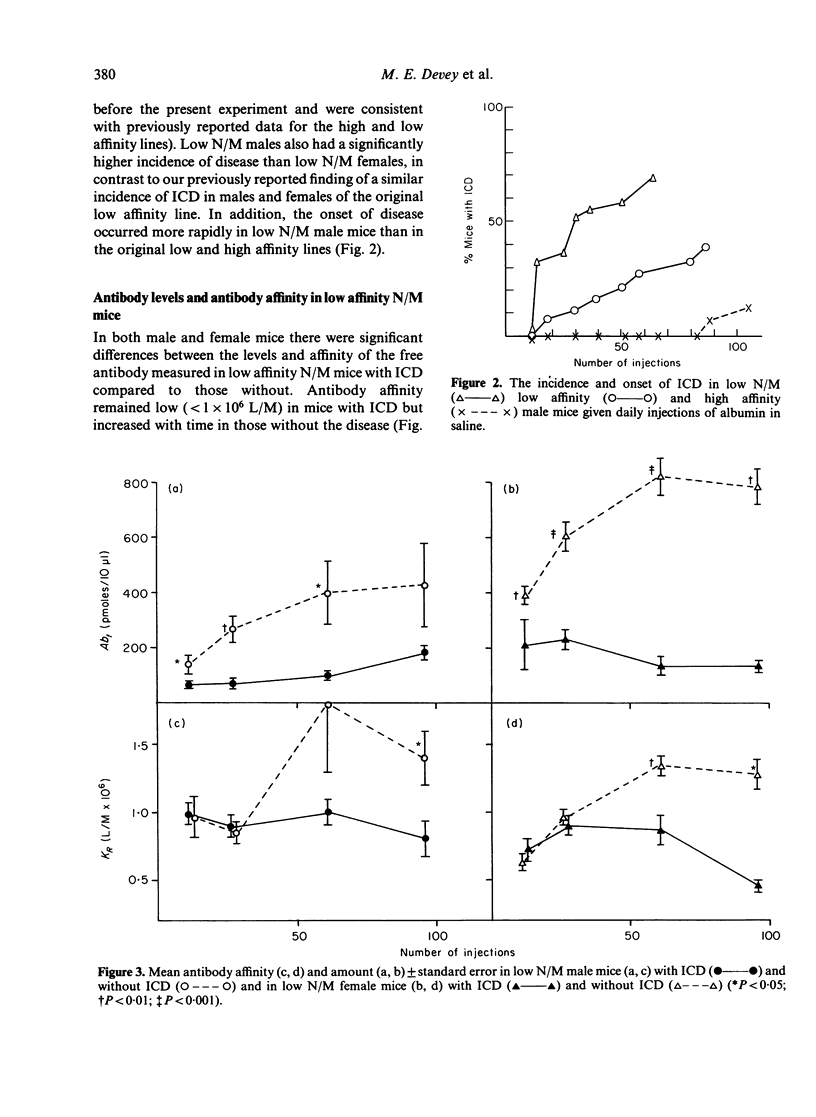
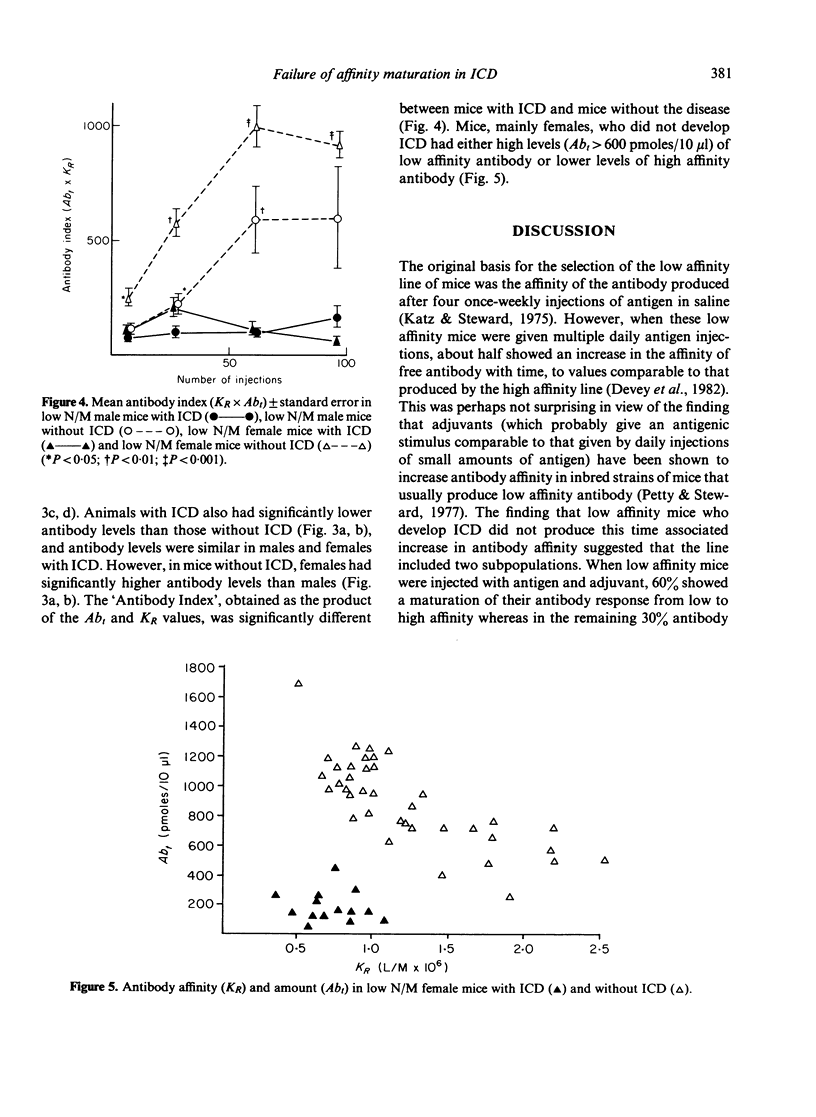
Mice, previously selected for the production of low affinity antibody after four injections of protein antigens in saline, fell into two groups on the basis of their antibody response after injection of adjuvantized antigen. One group produced antibody of sequentially rising affinity but the other produced only low affinity antibody. Mice from the latter group were interbred to produce low affinity non-maturing mice (low N/M mice). Daily injections in these mice produced a more rapid and severe glomerulonephritis than that observed in mice of the original low affinity line. Male low N/M mice were more severely affected than female low N/M mice. Susceptibility to the disease were associated not only with an inability to produce the maturational transition from low affinity to high affinity antibody with time but also with the production of low levels of antibody. It is suggested that these quantitative and qualitative defects in the antibody response may lead to increased susceptibility to immune complex disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers J. H., Steward M. W., Soothill J. F. Differences in immune elimination in inbred mice. The role of low affinity antibody. Clin Exp Immunol. 1972 Sep;12(1):121–132. [PMC free article] [PubMed] [Google Scholar]
- Devey M. E., Bleasdale K., Collins M., Steward M. W. Experimental antigen-antibody complex disease in mice. The role of antibody levels, antibody affinity and circulating antigen-antibody complexes. Int Arch Allergy Appl Immunol. 1982;68(1):47–53. doi: 10.1159/000233066. [DOI] [PubMed] [Google Scholar]
- Devey M. E., Steward M. W. The induction of chronic antigen-antibody complex disease in selectively bred mice producing either high or low affinity antibody to protein antigens. Immunology. 1980 Oct;41(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- Gaze S., West N. J., Steward M. W. The use of a double isotope method in the determination of antibody affinity. J Immunol Methods. 1973 Dec;3(4):357–364. doi: 10.1016/0022-1759(73)90037-9. [DOI] [PubMed] [Google Scholar]
- Germuth F. G., Jr, Rodriguez E., Lorelle C. A., Trump E. I., Milano L. L., Wise O. Passive immune complex glomerulonephritis in mice: models for various lesions found in human disease. II. Low avidity complexes and diffuse proliferative glomerulonephritis with subepithelial deposits. Lab Invest. 1979 Oct;41(4):366–371. [PubMed] [Google Scholar]
- Germuth F. G., Rodriguez E., Wise O. L. Passive immune complex glomerulonephritis in mice. III. Clearance kinetics and properties of circulating complexes. Lab Invest. 1982 May;46(5):515–519. [PubMed] [Google Scholar]
- Haakenstad A. O., Striker G. E., Mannik M. The disappearance kinetics and glomerular deposition of small-latticed soluble immune complexes. Immunology. 1982 Nov;47(3):407–414. [PMC free article] [PubMed] [Google Scholar]
- Katz F. E., Steward M. W. The genetic control of antibody affinity in mice. Immunology. 1975 Sep;29(3):543–548. [PMC free article] [PubMed] [Google Scholar]
- Koyama A., Niwa Y., Shigematsu H., Taniguchi M., Tada T. Studies on passive serum sickness. II. Factors determining the localization of antigen-antibody complexes in the murine renal glomerulus. Lab Invest. 1978 Mar;38(3):253–262. [PubMed] [Google Scholar]
- Steward M. W. Chronic immune complex disease in mice: the role of antibody affinity. Clin Exp Immunol. 1979 Dec;38(3):414–423. [PMC free article] [PubMed] [Google Scholar]


