Abstract
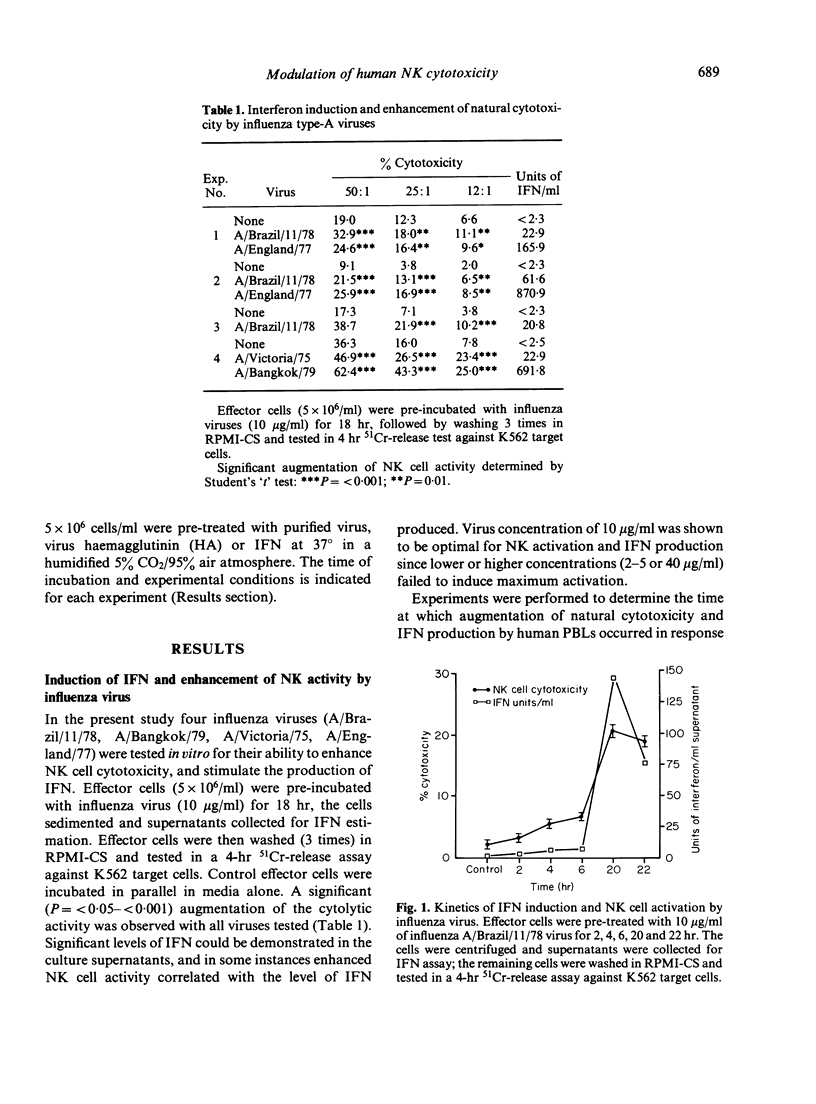
The influence of intact influenza virus and purified detergent solubilized haemagglutinin (HA) subunits from these viruses on human natural killer (NK) cell activity was examined. Effector cells incubated with whole influenza virus for 18 hr initiated the production of alpha interferon which was associated with the enhancement of NK cell activity. In contrast, purified influenza virus HA suppressed NK activity in a dose-dependent manner, when added at the onset of the cytotoxicity assay, or when used to pre-treated effector cells prior to assay for cytotoxicity against K562 target cells. Effector cells exposed to influenza HA for 90 min, washed and re-incubated in fresh medium for up to 18 hr, failed to regain their cytotoxicity. Suppression of NK cell cytotoxicity could not be ascribed to direct toxicity of HA preparations or residual detergent and preservative in these preparations. The augmented cytotoxicity of activated human effector cells was also susceptible to suppression by virus HA, and pretreatment of human PBL effector cells with HA for 90 min, prior to exposure to human alpha interferon caused NK effector cells to become refractive to the enhancing effects of HIFN. That direct interaction between influenza virus HA and effector cells was a requirement for suppression of activity was shown in experiments using Bromelain-released influenza HA, which would not be expected to bind to cells and which failed to suppress NK cell activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. S., Giebink G. S., Mills E. L., Quie P. G. Polymorphonuclear leukocyte dysfunction during influenza virus infection in chinchillas. J Infect Dis. 1981 Jun;143(6):836–845. doi: 10.1093/infdis/143.6.836. [DOI] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Brunda M. J., Herberman R. B., Holden H. T. Inhibition of murine natural killer cell activity by prostaglandins. J Immunol. 1980 Jun;124(6):2682–2687. [PubMed] [Google Scholar]
- Casali P., Sissons J. G., Buchmeier M. J., Oldstone M. B. In vitro generation of human cytotoxic lymphocytes by virus. Viral glycoproteins induce nonspecific cell-mediated cytotoxicity without release of interferon. J Exp Med. 1981 Sep 1;154(3):840–855. doi: 10.1084/jem.154.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate T. R., Couch R. B. Lack of effect of influenza virus infection on induction and expression of delayed hypersensitivity. J Infect Dis. 1981 Sep;144(3):280–280. doi: 10.1093/infdis/144.3.280. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Stocks N., Zoon K., Stanton G. J., Timonen T., Herberman R. B. Positive self regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes viruses. J Exp Med. 1982 Oct 1;156(4):1222–1234. doi: 10.1084/jem.156.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn S., Blomgren H., Strander H. Interferon and spontaneous cytotoxicity in man. V. Enhancement of spontaneous cytotoxicity in patients receiving human leukocyte interferon. Int J Cancer. 1980 Oct 15;26(4):419–428. doi: 10.1002/ijc.2910260406. [DOI] [PubMed] [Google Scholar]
- Ennis F. A., Meager A., Beare A. S., Qi Y. H., Riley D., Schwarz G., Schild G. C., Rook A. H. Interferon induction and increased natural killer-cell activity in influenza infections in man. Lancet. 1981 Oct 24;2(8252):891–893. doi: 10.1016/s0140-6736(81)91390-8. [DOI] [PubMed] [Google Scholar]
- Ennis F. A., Meager A. Immune interferon produced to high levels by antigenic stimulation of human lymphocytes with influenza virus. J Exp Med. 1981 Nov 1;154(5):1279–1289. doi: 10.1084/jem.154.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faguet G. B. The effect of killed influenza virus vaccine on the kinetics of normal human lymphocytes. J Infect Dis. 1981 Feb;143(2):252–258. doi: 10.1093/infdis/143.2.252. [DOI] [PubMed] [Google Scholar]
- Flanagan M. T., Skehel J. J. The conformation of influenza virus haemagglutinin. FEBS Lett. 1977 Aug 1;80(1):57–60. doi: 10.1016/0014-5793(77)80406-7. [DOI] [PubMed] [Google Scholar]
- Goldfarb R. H., Herberman R. B. Natural killer cell reactivity: regulatory interactions and among phorbol ester, interferon, cholera toxin, and retinoic acid. J Immunol. 1981 Jun;126(6):2129–2135. [PubMed] [Google Scholar]
- Green J. A., Charette R. P., Yeh T. J., Smith C. B. Presence of interferon in acute- and convalescent-phase sera of humans with influenza or influenza-like illness of undetermined etiology. J Infect Dis. 1982 Jun;145(6):837–841. doi: 10.1093/infdis/145.6.837. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Pollard R. B., Lutwick L. I., Gregory P. B., Robinson W. S., Merigan T. C. Effect of human leukocyte interferon on hepatitis B virus infection in patients with chronic active hepatitis. N Engl J Med. 1976 Sep 2;295(10):517–522. doi: 10.1056/NEJM197609022951001. [DOI] [PubMed] [Google Scholar]
- Hassan Z. M., Rees R. C. Natural antitumour cytotoxicity of mouse lymph node cells; characterization and specificity studies. Eur J Cancer. 1980 Sep;16(9):1263–1270. doi: 10.1016/0014-2964(80)90187-5. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Härfast B., Orvell C., Alsheikhly A., Andersson T., Perlmann P., Norrby E. The role of viral glycoproteins in mumps-virus-dependent lymphocyte-mediated cytotoxicity in vitro. Scand J Immunol. 1980;11(4):391–400. doi: 10.1111/j.1365-3083.1980.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Jarstrand C., Wasserman J., Dahl G. Peripheral blood lymphocyte populations in influenza patients. Scand J Infect Dis. 1977;9(1):1–3. doi: 10.3109/inf.1977.9.issue-1.01. [DOI] [PubMed] [Google Scholar]
- Johnston M. D. The characteristics required for a Sendai virus preparation to induce high levels of interferon in human lymphoblastoid cells. J Gen Virol. 1981 Sep;56(Pt 1):175–184. doi: 10.1099/0022-1317-56-1-175. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Induction of natural killer cells during murine influenza virus infection. Immunobiology. 1981;160(3-4):352–366. doi: 10.1016/s0171-2985(81)80061-7. [DOI] [PubMed] [Google Scholar]
- Manzella J. P., Roberts N. J., Jr Human macrophage and lymphocyte responses to mitogen stimulation after exposure to influenza virus, ascorbic acid, and hyperthermia. J Immunol. 1979 Nov;123(5):1940–1944. [PubMed] [Google Scholar]
- Oxford J. S., Hockley D. J., Heath T. D., Patterson S. The interaction of influenza virus haemagglutinin with phospholipid vesicles - morphological and immunological studies. J Gen Virol. 1981 Feb;52(Pt 2):329–343. doi: 10.1099/0022-1317-52-2-329. [DOI] [PubMed] [Google Scholar]
- Oxford J. S., Schild G. C. Quantification of influenza virus structural proteins using rocket immunoelectrophoresis. J Gen Virol. 1978 Jan;38(1):187–193. doi: 10.1099/0022-1317-38-1-187. [DOI] [PubMed] [Google Scholar]
- Reed W. P., Olds J. W., Kisch A. L. Decreased skin-test reactivity associated with influenza. J Infect Dis. 1972 Apr;125(4):398–402. doi: 10.1093/infdis/125.4.398. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Klein M. Target-effector interaction in the natural killer cell system. IV. Modulation by cyclic nucleotides. J Immunol. 1979 Dec;123(6):2785–2790. [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Koprowski H. Cell-mediated cytotoxicity against virus-infected target cells in humans. II. Interferon induction and activation of natural killer cells. J Immunol. 1978 Aug;121(2):532–538. [PubMed] [Google Scholar]
- Skehel J. J., Schild G. C. The polypeptide composition of influenza A viruses. Virology. 1971 May;44(2):396–408. doi: 10.1016/0042-6822(71)90270-4. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Doe W. F. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice: natural killer cell activity in cultured spleen leukocytes concomitant with T-cell-dependent immune interferon production. Infect Immun. 1980 Nov;30(2):473–483. doi: 10.1128/iai.30.2.473-483.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley N. G., Laver W. G., Downie J. C. Binding of antibodies to isolated haemagglutinin and neuraminidase molecules of influenza virus observed in the electron microscope. J Mol Biol. 1977 Jan 25;109(3):405–421. doi: 10.1016/s0022-2836(77)80020-x. [DOI] [PubMed] [Google Scholar]


