Abstract
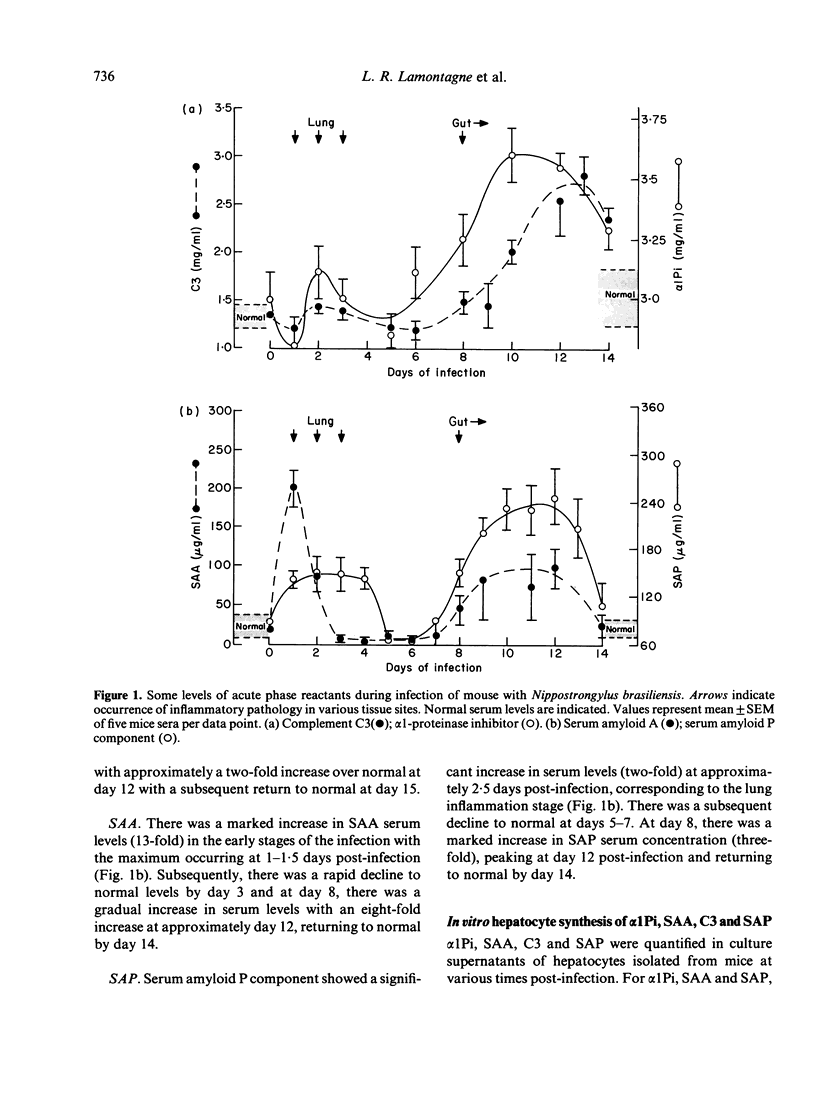
Systemic inflammatory reactions are a prominent feature of many parasitic infections and the cellular and humoral components of the acute phase reaction may have an impact on the host-parasite relationship. We examined serum changes of four acute phase reactants: alpha 1-proteinase inhibition (alpha 1Pi); complement C3; serum amyloid A protein (SAA); and serum amyloid P component (SAP), in mice undergoing a primary infection with Nippostrongylus brasiliensis. SAA and SAP showed changes within the first 2 days of infection indicating the presence of an acute phase response associated with inflammation in the lung. Alpha 1Pi and C3 serum levels were not altered. However, all four acute phase reactants were synthesized in greater amounts by primary cultures of hepatocytes taken from infected animals at this time. Subsequently, as parasite-mediated inflammatory changes occur in the gut, both serum and hepatocyte cultures demonstrate an acute inflammatory response in all four reactants. It is proposed that the early reaction between parasites and macrophage/monocyte lead to the release of a mediator of inflammation which initiates the hepatocyte response. In this infection, at least one of the APR is shown to localize to the site of inflammation influencing the host-parasite relationship.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldo-Benson M. A., Benson M. D. SAA suppression of immune response in vitro: evidence for an effect on T cell-macrophage interaction. J Immunol. 1982 Jun;128(6):2390–2392. [PubMed] [Google Scholar]
- Arora P. K., Miller H. C., Aronson L. D. alpha1-Antitrypsin is an effector of immunological stasis. Nature. 1978 Aug 10;274(5671):589–590. doi: 10.1038/274589a0. [DOI] [PubMed] [Google Scholar]
- Befus A. D., Johnston N., Bienenstock J. Nippostrongylus brasiliensis: mast cells and histamine levels in tissues of infected and normal rats. Exp Parasitol. 1979 Aug;48(1):1–8. doi: 10.1016/0014-4894(79)90048-1. [DOI] [PubMed] [Google Scholar]
- Benson M. D., Aldo-Benson M. Effect of purified protein SAA on immune response in vitro: mechanisms of suppression. J Immunol. 1979 May;122(5):2077–2082. [PubMed] [Google Scholar]
- Benson M. D., Kleiner E. Synthesis and secretion of serum amyloid protein A (SAA) by hepatocytes in mice treated with casein. J Immunol. 1980 Feb;124(2):495–499. [PubMed] [Google Scholar]
- Bornstein D. L., Walsh E. C. Endogenous mediators of the acute-phase reaction. I. Rabbit granulocytic pyrogen and its chromatographic subfractions. J Lab Clin Med. 1978 Feb;91(2):236–245. [PubMed] [Google Scholar]
- Courtoy P. J., Lombart C., Feldmann G., Moguilevsky N., Rogier E. Synchronous increase of four acute phase proteins synthesized by the same hepatocytes during the inflammatory reaction: a combined biochemical and morphologic kinetics study in the rat. Lab Invest. 1981 Feb;44(2):105–115. [PubMed] [Google Scholar]
- Croll N. A., Ma K. The location of parasites within their hosts: the passage of Nippostrongylus brasiliensis through the lungs of the laboratory rat. Int J Parasitol. 1978 Aug;8(4):289–295. doi: 10.1016/0020-7519(78)90093-0. [DOI] [PubMed] [Google Scholar]
- Deschenes J., Valet J. P., Marceau N. Hepatocytes from newborn and weanling rats in monolayer culture: isolation by perfusion, fibronectin-mediated adhesion, spreading, and functional activities. In Vitro. 1980 Aug;16(8):722–730. doi: 10.1007/BF02619202. [DOI] [PubMed] [Google Scholar]
- Egwang T. G., Gauldie J., Befus D. Complement-dependent killing of Nippostrongylus brasiliensis infective larvae by rat alveolar macrophages. Clin Exp Immunol. 1984 Jan;55(1):149–156. [PMC free article] [PubMed] [Google Scholar]
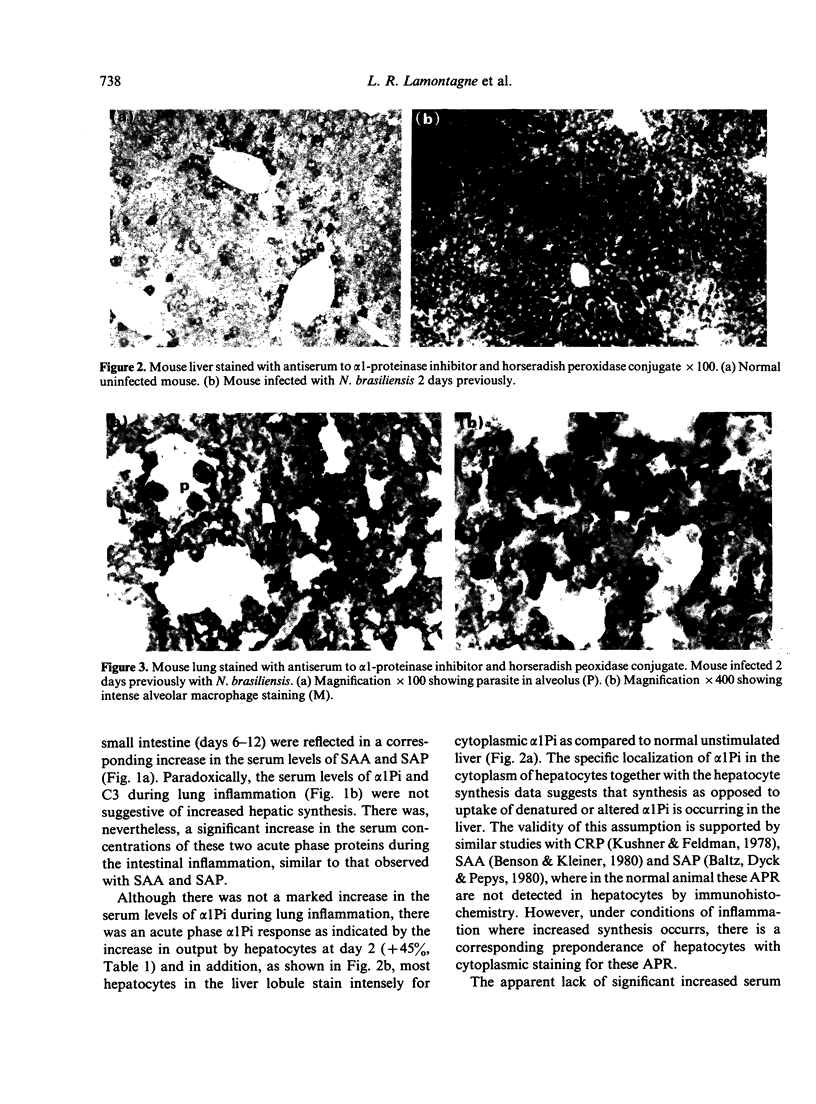
- Gauldie J., Lamontagne L., Horsewood P., Jenkins E. Immunohistochemical localization of alpha 1-antitrypsin in normal mouse liver and pancreas. Am J Pathol. 1980 Dec;101(3):723–736. [PMC free article] [PubMed] [Google Scholar]
- Hudig D., Sell S. Serum concentrations of alpha-macrofeto-protein (acute-phase alpha2-macroglobulin), a proteinase inhibitor, in pregnant and neonatal rats and in rats with acute inflammation. Inflammation. 1978 Jun;3(2):137–148. doi: 10.1007/BF00910735. [DOI] [PubMed] [Google Scholar]
- James K., Hansen B., Gewurz H. Binding of C-reactive protein to human lymphocytes. I. Requirement for a binding specificity. J Immunol. 1981 Dec;127(6):2539–2544. [PubMed] [Google Scholar]
- James K., Hansen B., Gewurz H. Binding of C-reactive protein to human lymphocytes. II. Interaction with a subset of cells bearing the Fc receptor. J Immunol. 1981 Dec;127(6):2545–2550. [PubMed] [Google Scholar]
- Kinsella T. D., Fritzler M. J. CRP: an immunoregulatory protein. J Rheumatol. 1980 May-Jun;7(3):272–274. [PubMed] [Google Scholar]
- Koj A., Regoeczi E. Effect of experimental inflammation on the synthesis and distribution of antithrombin III and alpha1-antitrypsin in rabbits. Br J Exp Pathol. 1978 Oct;59(5):473–481. [PMC free article] [PubMed] [Google Scholar]
- Kushner I., Feldmann G. Control of the acute phase response. Demonstration of C-reactive protein synthesis and secretion by hepatocytes during acute inflammation in the rabbit. J Exp Med. 1978 Aug 1;148(2):466–477. doi: 10.1084/jem.148.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le P. T., Muller M. T., Mortensen R. F. Acute phase reactants of mice. I. Isolation of serum amyloid P-component (SAP) and its induction by a monokine. J Immunol. 1982 Aug;129(2):665–672. [PubMed] [Google Scholar]
- Mackenzie C. D., Jungery M., Taylor P. M., Ogilvie B. M. Activation of complement, the induction of antibodies to the surface of nematodes and the effect of these factors and cells on worm survival in vitro. Eur J Immunol. 1980 Aug;10(8):594–601. doi: 10.1002/eji.1830100805. [DOI] [PubMed] [Google Scholar]
- McAdam K. P., Sipe J. D. Murine model for human secondary amyloidosis: genetic variability of the acute-phase serum protein SAA response to endotoxins and casein. J Exp Med. 1976 Oct 1;144(4):1121–1127. doi: 10.1084/jem.144.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman C. R., Pulliam L. A., Kampschmidt R. F. Comparison of leukocytic pyrogen and leukocytic endogenous mediator. Proc Soc Exp Biol Med. 1977 Feb;154(2):224–227. doi: 10.3181/00379727-154-39642. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F. C-Reactive protein (CRP)-mediated inhibition of the induction of in vitro antibody formation. I. T-cell dependence of the inhibition. Cell Immunol. 1979 May;44(2):270–282. doi: 10.1016/0008-8749(79)90005-4. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F. Inhibition of the polyclonal antibody plaque-forming cell: response of human B lymphocytes by C-reactive protein (CRP) and CRP complexes. Cell Immunol. 1982 Jan 1;66(1):99–110. doi: 10.1016/0008-8749(82)90161-7. [DOI] [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Oppenheim J. J., Stadler B. M., Siraganian R. P., Mage M., Mathieson B. Lymphokines: their role in lymphocyte responses. Properties of interleukin 1. Fed Proc. 1982 Feb;41(2):257–262. [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M., Gomer K., Davies A. J., Doenhoff M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979 Mar 15;278(5701):259–261. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B. Isolation of serum amyloid P-component (protein SAP) in the mouse. Immunology. 1979 Jul;37(3):637–641. [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A., Rosenthal A. S. Adherent cell function in murine T-lymphocyte antigen recognition. IV. Enhancement of murine T-cell antigen recognition by human leukocytic pyrogen. J Exp Med. 1979 Sep 19;150(3):709–714. doi: 10.1084/jem.150.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYMONS L. E. KINETICS OF THE EPITHELIAL CELLS, AND MORPHOLOGY OF VILLI AND CRYPTS IN THE JEJUNUM OF THE RAT INFECTED BY THE NEMATODE NIPPOSTRONGYLUS BRASILIENSIS. Gastroenterology. 1965 Aug;49:158–168. [PubMed] [Google Scholar]
- Scharfstein J., Barcinski M. A., Leon L. L. Induction of the acute-phase protein serum amyloid P in experimental Chagas' disease. Infect Immun. 1982 Jan;35(1):46–51. doi: 10.1128/iai.35.1.46-51.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger M. J., McAdam K. P., Kaplan M. M., Sipe J. D., Vogel S. N., Rosenstreich D. L. Monokine-induced synthesis of serum amyloid A protein by hepatocytes. Nature. 1980 Jun 12;285(5765):498–500. doi: 10.1038/285498a0. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., McAdam K. P., Torain B. F., Pollock P. S. Isolation and structural properties of murine SAA--the acute phase serum precursor of amyloid AA. Immunol Commun. 1977;6(1):1–12. doi: 10.3109/08820137709055799. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., Vogel S. N., Ryan J. L., McAdam K. P., Rosenstreich D. L. Detection of a mediator derived from endotoxin-stimulated macrohpages that induces the acute phase serum amyloid A response in mice. J Exp Med. 1979 Sep 19;150(3):597–606. doi: 10.1084/jem.150.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirica A. E., Richards W., Tsukada Y., Sattler C. A., Pitot H. C. Fetal phenotypic expression by adult rat hepatocytes on collagen gel/nylon meshes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):283–287. doi: 10.1073/pnas.76.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztein M. B., Vogel S. N., Sipe J. D., Murphy P. A., Mizel S. B., Oppenheim J. J., Rosenstreich D. L. The role of macrophages in the acute-phase response: SAA inducer is closely related to lymphocyte activating factor and endogenous pyrogen. Cell Immunol. 1981 Sep 1;63(1):164–176. doi: 10.1016/0008-8749(81)90037-x. [DOI] [PubMed] [Google Scholar]
- Takasugi M. An improved fluorochromatic cytotoxic test. Transplantation. 1971 Aug;12(2):148–151. doi: 10.1097/00007890-197108000-00010. [DOI] [PubMed] [Google Scholar]
- Vetter M. L., Gewurz H., Hansen B., James K., Baum L. L. Effects of C-reactive protein on human lymphocyte responsiveness. J Immunol. 1983 May;130(5):2121–2126. [PubMed] [Google Scholar]
- WESCOTT R. B., TODD A. C. A COMPARISON OF THE DEVELOPMENT OF NIPPOSTRONGYLUS BRASILIENSIS IN GERM-FREE AND CONVENTIONAL MICE. J Parasitol. 1964 Feb;50:138–143. [PubMed] [Google Scholar]




