Abstract
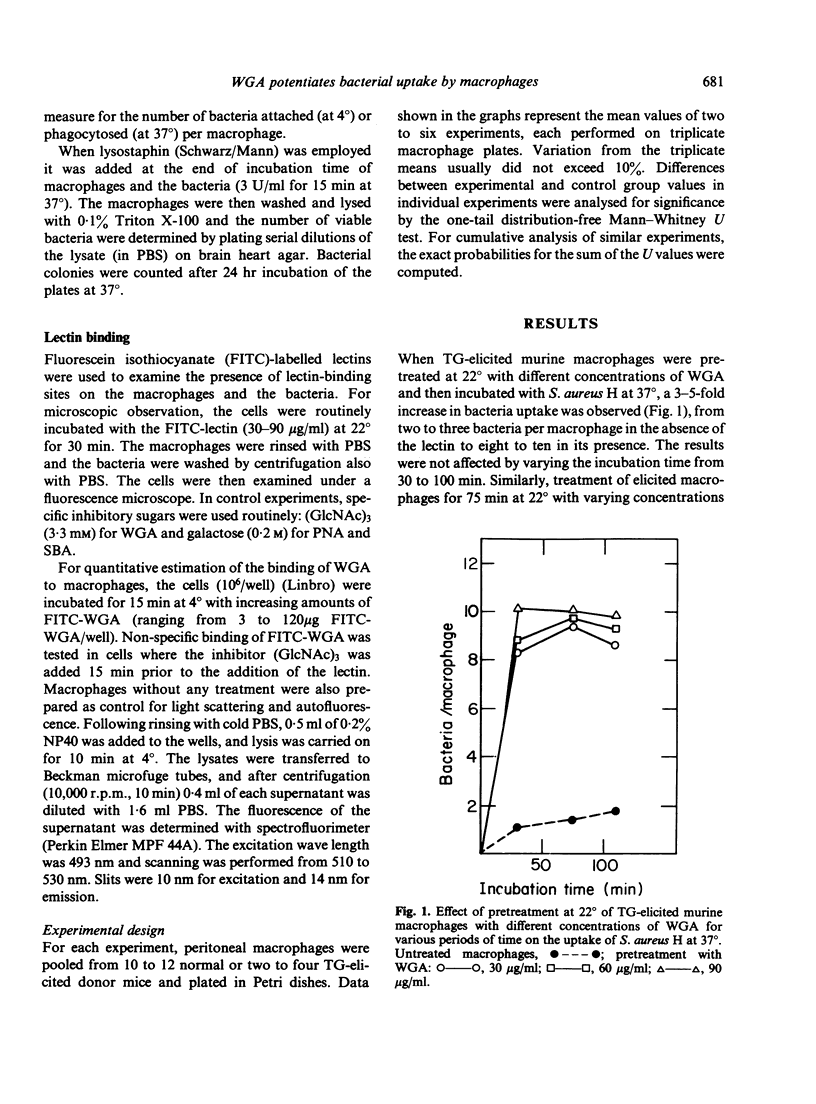
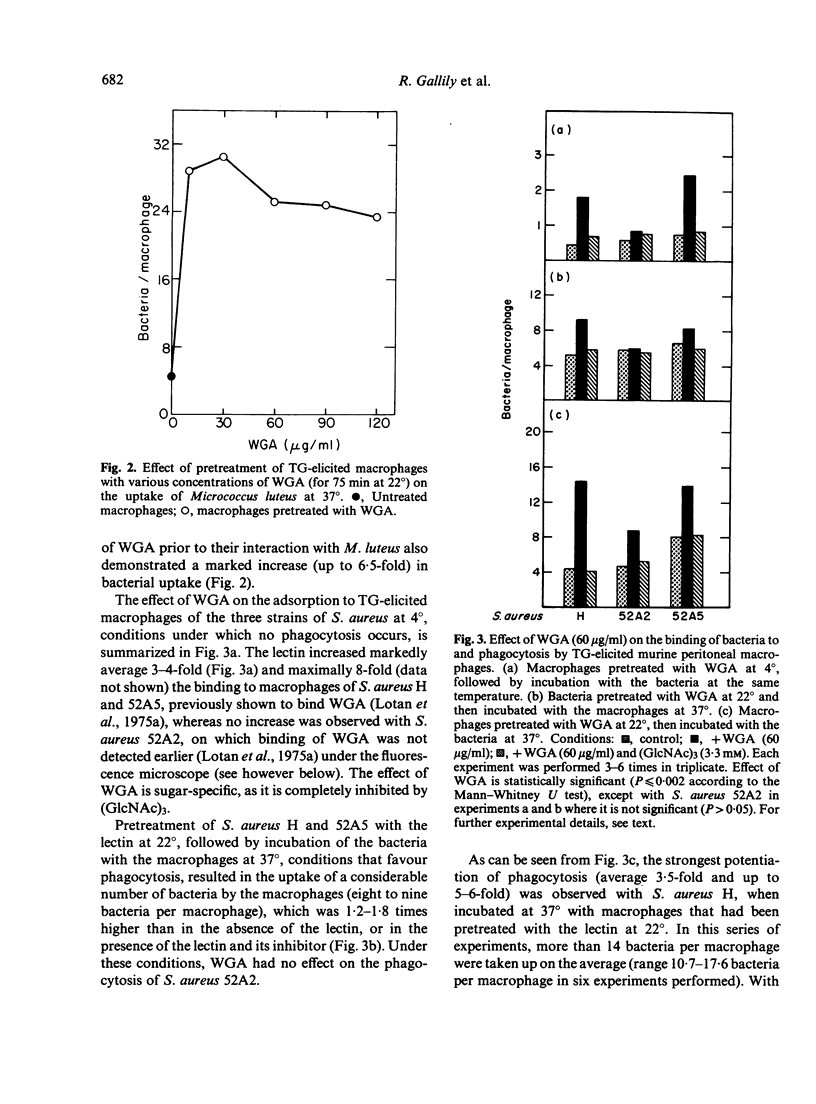
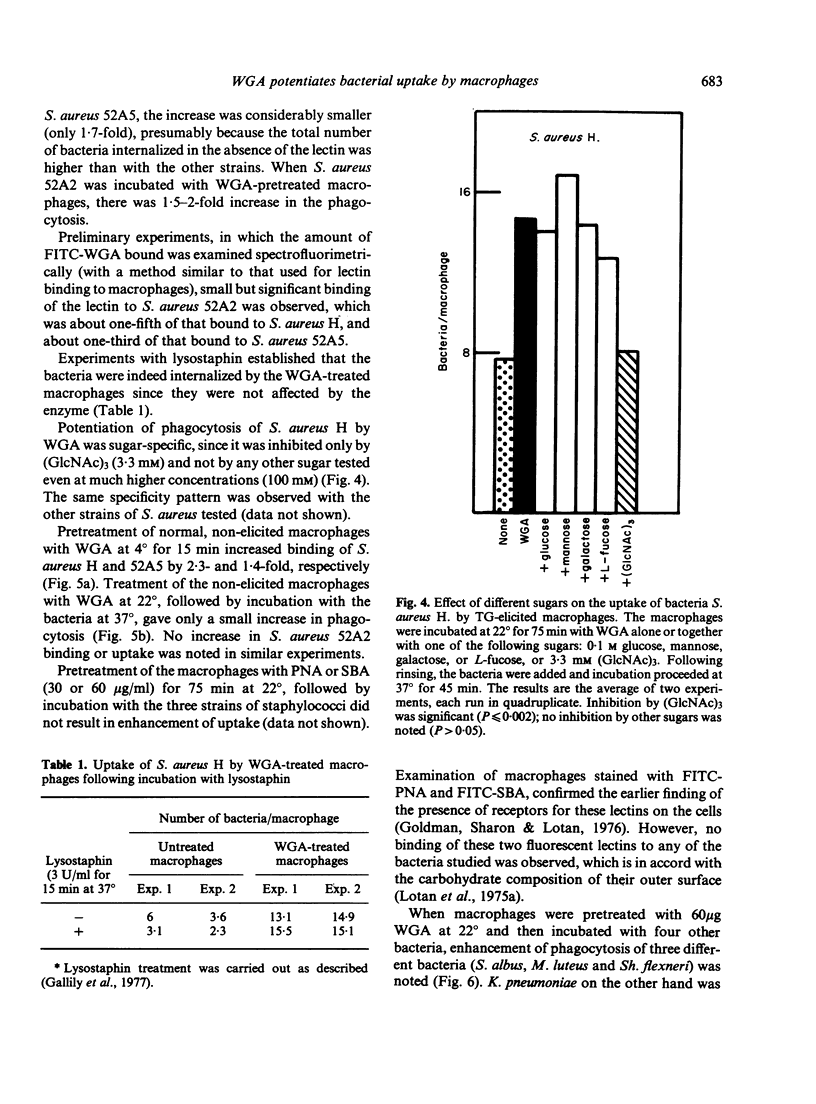
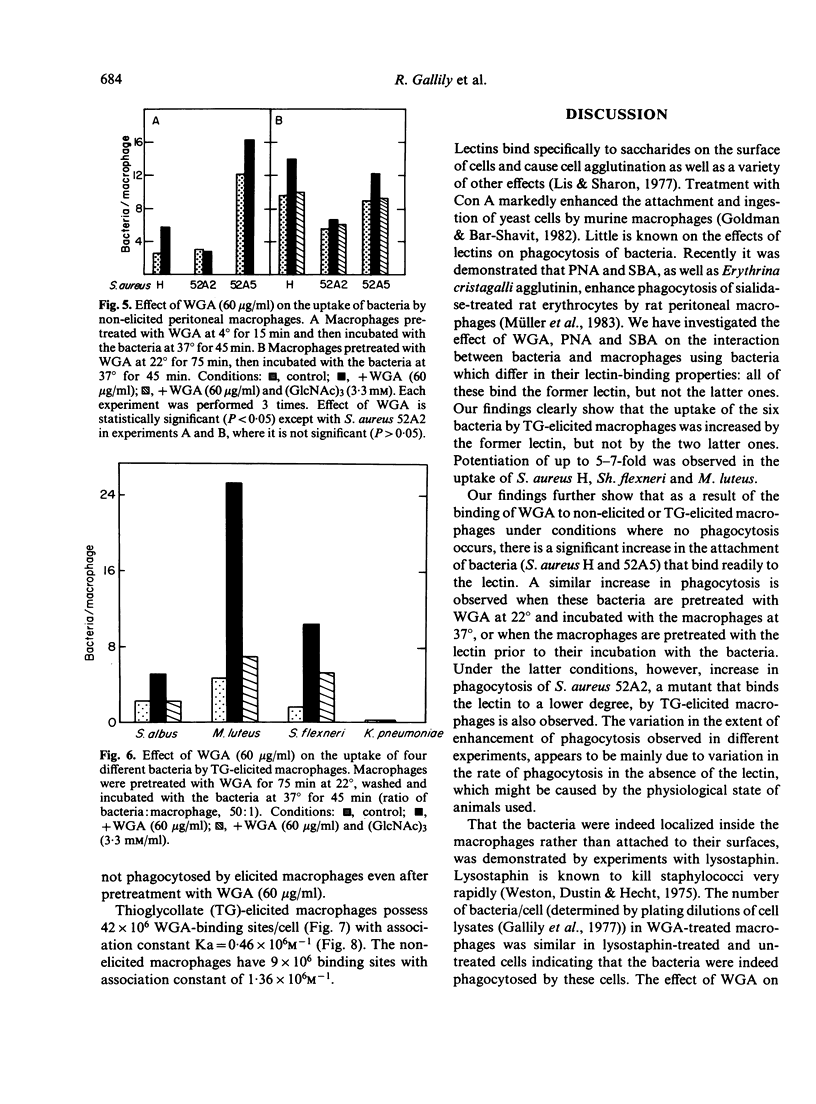
Exposure of thioglycollate-elicited murine peritoneal macrophages to wheat germ agglutinin (WGA) increased markedly the uptake of six different bacteria, which have surface receptors for the lectin. Uptake of Staphylococcus aureus H was higher by 3-5-fold, of S. aureus 52A2 by 1.8-fold, of S. aureus 52A5 by 1.7-fold, of S. albus by 2.3-fold, of Shigella flexneri by 6-fold and of Micrococcus luteus by 6.5-fold. Klebsiella pneumoniae, devoid of receptors for WGA, was not phagocytosed following pretreatment of macrophages with the lectin. Pretreatment of the bacteria with the lectin also resulted, in most cases, in an increase in phagocytosis. Interaction of WGA with the macrophages and with the bacteria, as well as the potentiation of phagocytosis, was abolished by tri-N-acetylchitotriose, a saccharide that binds specifically to WGA, but not by monosaccharides which do not interact with this lectin. With non-elicited macrophages, enhancement of phagocytosis by WGA was less pronounced, probably because of the higher number of lectin-binding sites (5-fold) on the elicited cells. Peanut agglutinin and soybean agglutinin, that bind to macrophages but not to the bacteria studied, lack the ability to potentiate phagocytosis. Macrophage surface sugars thus appear to play an important role in phagocytosis by serving as receptors for lectins that form bridges between the macrophages and the microorganisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Goldman R. Concanavalin A-mediated attachment and ingestion of yeast cells by macrophages. Exp Cell Res. 1976 May;99(2):221–236. doi: 10.1016/0014-4827(76)90578-4. [DOI] [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. N., Mirelman D., Singer H. J., Park J. T. Properties of a novel pleiotropic bacteriophage-resistant mutant of Staphylococcus aureus H. J Bacteriol. 1969 Nov;100(2):846–853. doi: 10.1128/jb.100.2.846-853.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallily R., Douchan Z., Weiss D. W. Potentiation of mouse peritoneal macrophage antibacterial functions by treatment of the donor animals with the methanol extraction residue fraction of tubercle bacilli. Infect Immun. 1977 Nov;18(2):405–411. doi: 10.1128/iai.18.2.405-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Gallily R. Effect of X-irradiation on various functions of murine macrophages. Clin Exp Immunol. 1974 Apr;16(4):643–655. [PMC free article] [PubMed] [Google Scholar]
- Goldman R., Sharon N., Lotan R. A differential response elicited in macrophages on interaction with lectins. Exp Cell Res. 1976 May;99(2):408–422. doi: 10.1016/0014-4827(76)90598-x. [DOI] [PubMed] [Google Scholar]
- Gordon Julius A., Blumberg Shmaryahu, Lis Halina, Sharon Nathan. Purification of soybean agglutinin by affinity chromatography On sepharose-N-epsilon-aminocaproyl-beta-D-galactopyranosylamine. FEBS Lett. 1972 Aug 1;24(2):193–196. doi: 10.1016/0014-5793(72)80765-8. [DOI] [PubMed] [Google Scholar]
- Lotan R., Gussin A. E., Lis H., Sharon N. Purification of wheat germ agglutinin by affinity chromatography on a sepharose-bound N-acetylglucosamine derivative. Biochem Biophys Res Commun. 1973 May 15;52(2):656–662. doi: 10.1016/0006-291x(73)90763-8. [DOI] [PubMed] [Google Scholar]
- Lotan R., Sharon N., Mirelman D. Interaction of wheat-germ agglutinin with bacterial cells and cell-wall polymers. Eur J Biochem. 1975 Jun 16;55(1):257–262. doi: 10.1111/j.1432-1033.1975.tb02158.x. [DOI] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Eisen H. N. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975 Dec 1;142(6):1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston W. L., Dustin R. D., Hecht S. K. Quantitative assays of human monocyte-macrophage function. J Immunol Methods. 1975 Sep;8(3):213–222. doi: 10.1016/0022-1759(75)90114-3. [DOI] [PubMed] [Google Scholar]


