Abstract
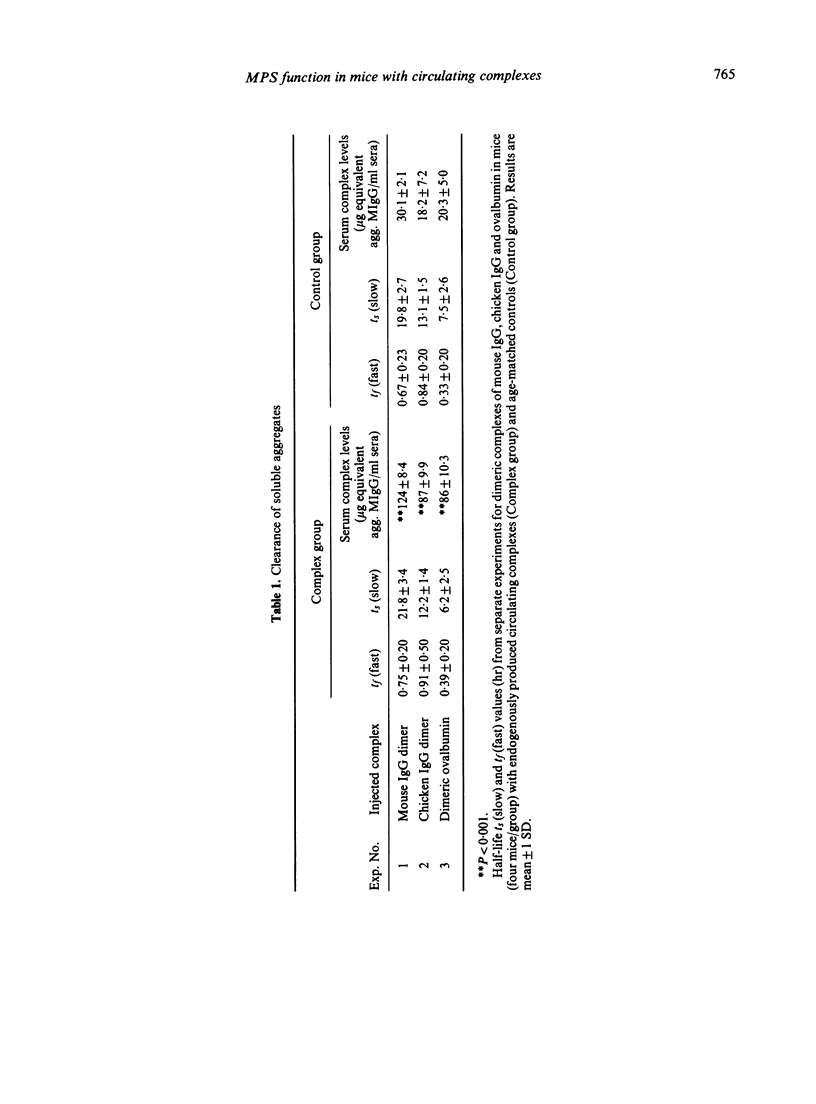
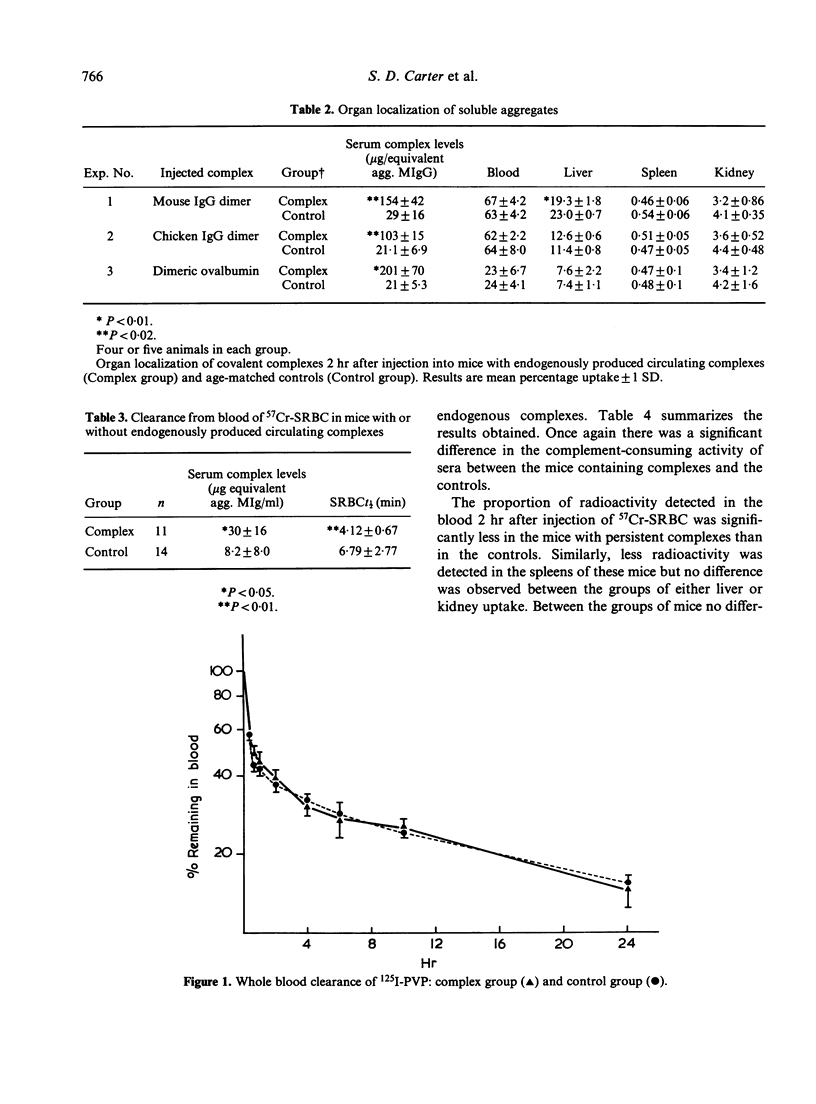
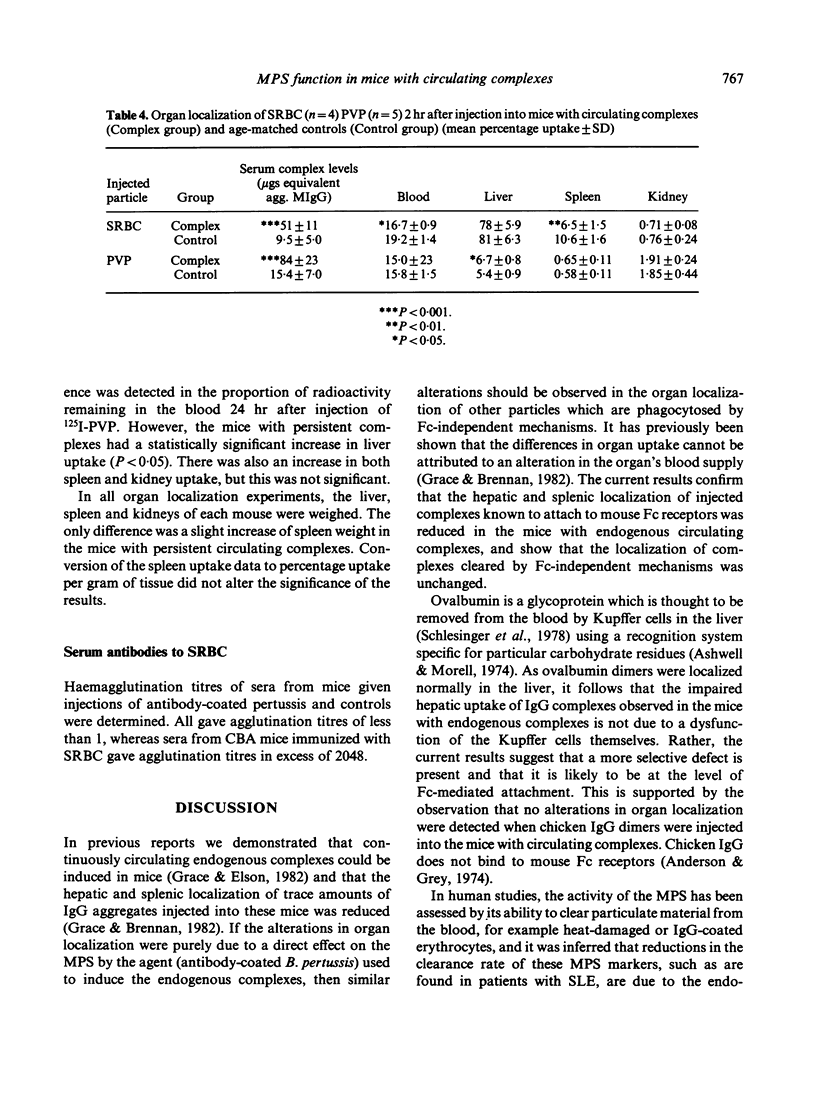
The clearance and organ localization of a number of substances cleared by either Fc-dependent or -independent mechanisms was studied in normal mice and in mice with endogenously produced persistent circulating complexes. Clearance of covalent dimers of mouse IgG, chicken IgG and ovalbumin were no different between the two groups of mice. By contrast, hepatic and splenic uptake of dimeric mouse IgG (but not of chicken IgG or ovalbumin dimer) was impaired in the mice with persisting complexes. Surprisingly the rate of clearance of sheep red blood cells (SRBC) was increased in mice with persisting complexes as was hepatic uptake of polyvinyl pyrrolidone. It is suggested that the mononuclear phagocytes of mice with persistent circulating complexes are non-specifically stimulated while their ability to take up soluble complexes by Fc-dependent attachment is selectively impaired.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Grey H. M. Receptors for aggregated IgG on mouse lymphocytes: their presence on thymocytes, thymus-derived, and bone marrow-derived lymphocytes. J Exp Med. 1974 May 1;139(5):1175–1188. doi: 10.1084/jem.139.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Grace S. A., Elson C. J. Preparation of covalent IgG complexes of defined size and their clearance from the circulation of mice. J Immunol Methods. 1983 Jan 28;56(2):149–158. doi: 10.1016/0022-1759(83)90406-4. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Hawkins D. Studies on circulating immune complexes. 3. Factors governing the ability of circulating complexes to localize in blood vessels. J Exp Med. 1968 Jan 1;127(1):137–154. doi: 10.1084/jem.127.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devey M. E., Steward M. W. The induction of chronic antigen-antibody complex disease in selectively bred mice producing either high or low affinity antibody to protein antigens. Immunology. 1980 Oct;41(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- Elson C. J., Jablonska K. F., Taylor R. B. Functional half-life of virgin and primed B lymphocytes. Eur J Immunol. 1976 Sep;6(9):634–638. doi: 10.1002/eji.1830060908. [DOI] [PubMed] [Google Scholar]
- Finbloom D. S., Abeles D., Rifai A., Plotz P. H. The specificity of uptake of model immune complexes and other protein aggregates by the murine reticuloendothelial system. J Immunol. 1980 Sep;125(3):1060–1065. [PubMed] [Google Scholar]
- Finbloom D. S., Plotz P. H. Studies of reticuloendothelial function in the mouse with model immune complexes. I. Serum clearance and tissue uptake in normal C3H mice. J Immunol. 1979 Oct;123(4):1594–1599. [PubMed] [Google Scholar]
- Finbloom D. S., Plotz P. H. Studies of reticuloendothelial function in the mouse with model immune complexes. II. Serum clearance, tissue uptake, and reticuloendothelial saturation in NZB/W mice. J Immunol. 1979 Oct;123(4):1600–1603. [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Frank M. M., Hamburger M. I., Lawley T. J., Kimberly R. P., Plotz P. H. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979 Mar 8;300(10):518–523. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- Grace S. A., Brennan F. M. Clearance and localization of immunoglobulin oligomers in mice with chronic circulating endogenous complexes. Immunology. 1982 Oct;47(2):221–226. [PMC free article] [PubMed] [Google Scholar]
- Grace S. A., Elson C. J., Coeshott C. M. Production of antibodies to host IgG after transfer of histocompatible cells primed to host allotype. Clin Exp Immunol. 1980 Feb;39(2):449–454. [PMC free article] [PubMed] [Google Scholar]
- Grace S. A., Elson C. J. Continuous production of anti-host IgG antibodies contained in circulating IgG-anti-IgG complexes. Immunology. 1982 Oct;47(2):289–296. [PMC free article] [PubMed] [Google Scholar]
- Haakenstad A. O., Mannik M. Saturation of the reticuloendothelial system with soluble immune complexes. J Immunol. 1974 May;112(5):1939–1948. [PubMed] [Google Scholar]
- Haakenstad A. O., Mannik M. The disappearance kinetics of soluble immune complexes prepared with reduced and alkylated antibodies and with intact antibodies in mice. Lab Invest. 1976 Sep;35(3):283–292. [PubMed] [Google Scholar]
- Micklem H. S., Anderson N., Ure J., Jones H. P. Long-term immunoglobulin G production by transplanted thymus cells. Eur J Immunol. 1976 Jun;6(6):425–429. doi: 10.1002/eji.1830060609. [DOI] [PubMed] [Google Scholar]
- Morgan A. G., Steward M. W. Macrophage clearance function and immune complex disease in New Zealand Black/White F1 hybrid mice. Clin Exp Immunol. 1976 Oct;26(1):133–136. [PMC free article] [PubMed] [Google Scholar]
- Morton J. I., Siegel B. V. Reticuloendothelial activity of New Zealand black mice. Proc Soc Exp Biol Med. 1970 Mar;133(3):1055–1059. doi: 10.3181/00379727-133-34624. [DOI] [PubMed] [Google Scholar]
- NIH conference. Pathophysiology of immune hemolytic anemia. Ann Intern Med. 1977 Aug;87(2):210–222. doi: 10.7326/0003-4819-87-2-210. [DOI] [PubMed] [Google Scholar]
- Pestel J., Joseph M., Dessaint J. P., Capron A. Macrophage triggering by aggregated immunoglobulins. I. Delayed effect of IgG aggregates or immune complexes. J Immunol. 1981 May;126(5):1887–1891. [PubMed] [Google Scholar]
- Schlesinger P. H., Doebber T. W., Mandell B. F., White R., DeSchryver C., Rodman J. S., Miller M. J., Stahl P. Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with beta-glucuronidase, N-acetyl-beta-D-glucosaminidase, ribonuclease B and agalacto-orosomucoid. Biochem J. 1978 Oct 15;176(1):103–109. doi: 10.1042/bj1760103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. M., Taurog J. D., Metzger H. Dimeric immunoglobulin E serves as a unit signal for mast cell degranulation. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward M. W. Chronic immune complex disease in mice: the role of antibody affinity. Clin Exp Immunol. 1979 Dec;38(3):414–423. [PMC free article] [PubMed] [Google Scholar]
- WEIGLE W. O. Elimination of antigen-antibody complexes from sera of rabbits. J Immunol. 1958 Sep;81(3):204–213. [PubMed] [Google Scholar]


