Abstract
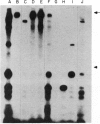
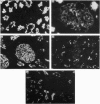
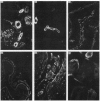
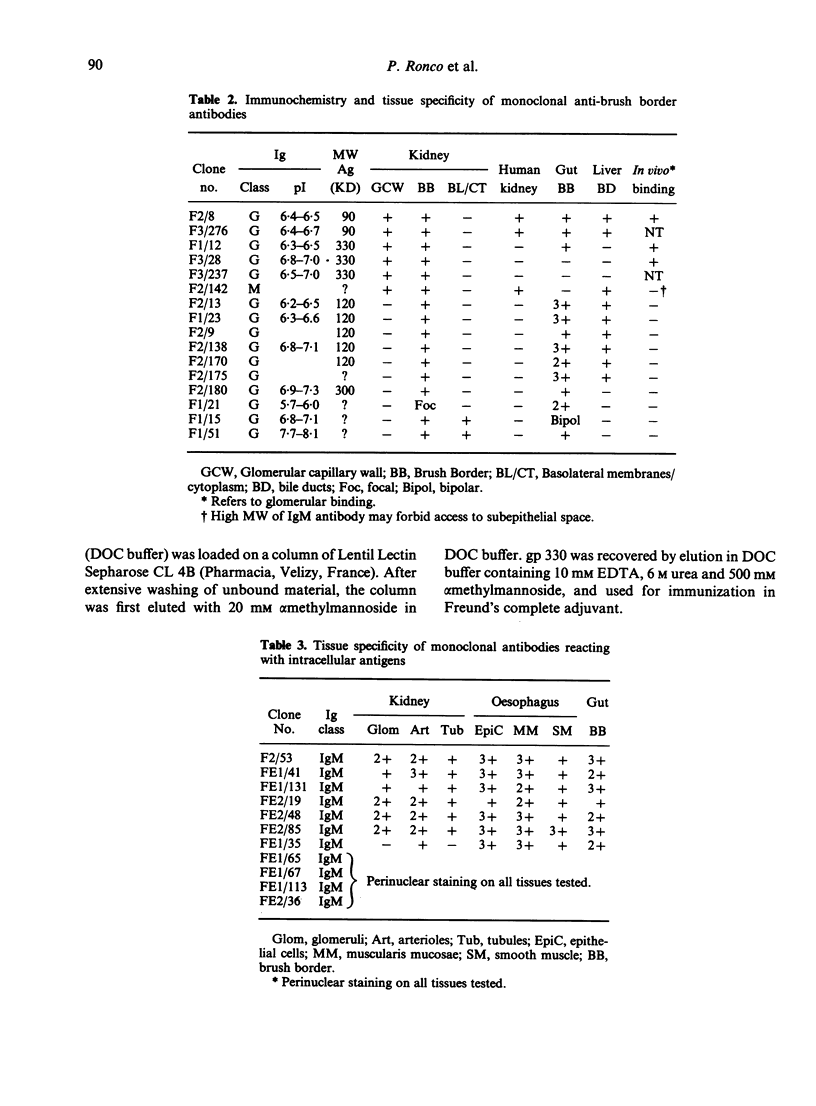
In order to understand further the processes involved in immunological injury of the kidney, we have prepared monoclonal antibodies against brush border (BB) antigens of rat proximal tubule. The 27 antibodies which constitute the basis of this report have been cloned, characterized immunochemically, and classified in three specificity groups on the basis of tissue reactivity. The first group is made up of six antibodies reacting with antigens simultaneously present on BB and glomerulus: three are directed against a high molecular weight (MW) protein which migrates with an apparent MW of 330,000; two react with a 90,000 MW protein that is present diffusely on renal and intestinal BB as well as on endothelial cells; one recognizes an antigen exclusively present on superficial tubules and glomerular epithelial cells, which could not be chemically characterized. The second group is made up of eight antibodies present on renal and intestinal BB: five react with a 120,000 MW antigen, one with a 300,000 MW antigen. The third group comprises 13 antibodies. Two are directed against antigens present within the cytoplasm or the basolateral membranes of renal tubules. Eleven react with intracellular antigens probably related to the cytoskeleton. Since they have been identified through several fusions, some of the monoclonal antibodies described are probably directed against immunodominant proteins of the BB. They open new possibilities for purifying the corresponding antigens by affinity chromatography as well as for obtaining BB preparations selectively depleted of the strongest immunogens thus favouring antibody production to previously unrecognized antigens.
Full text
PDF


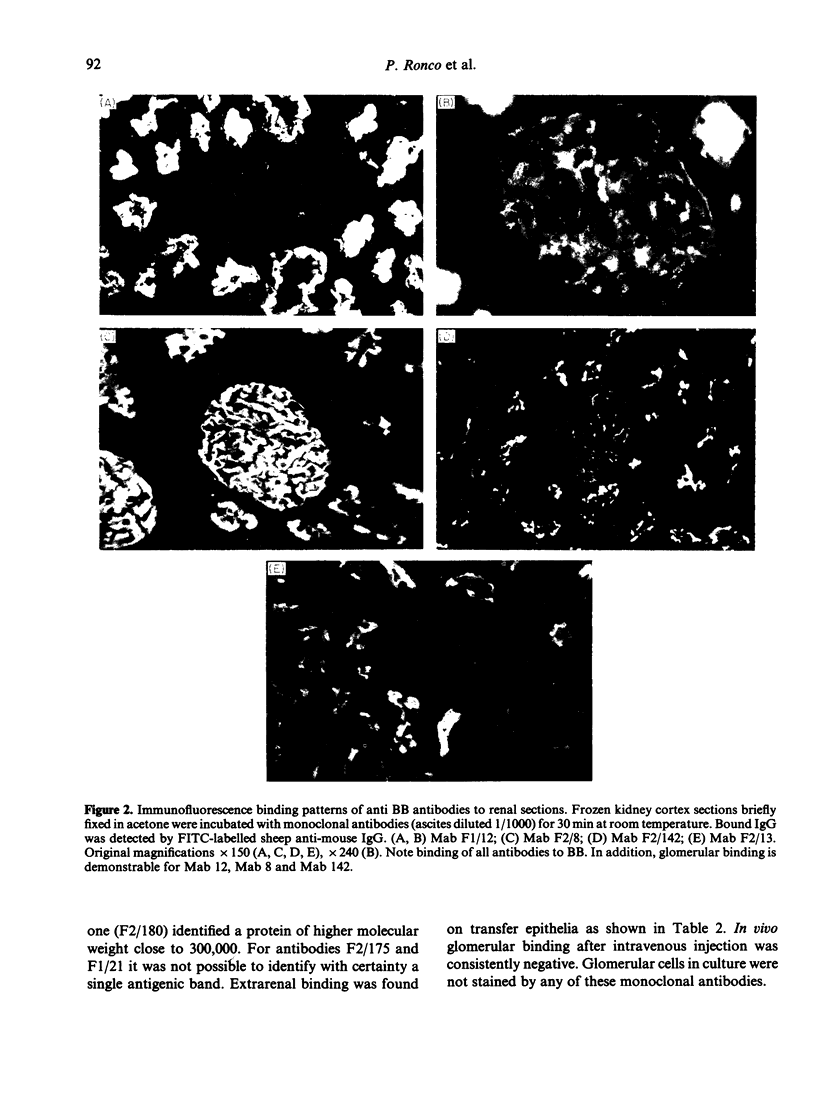
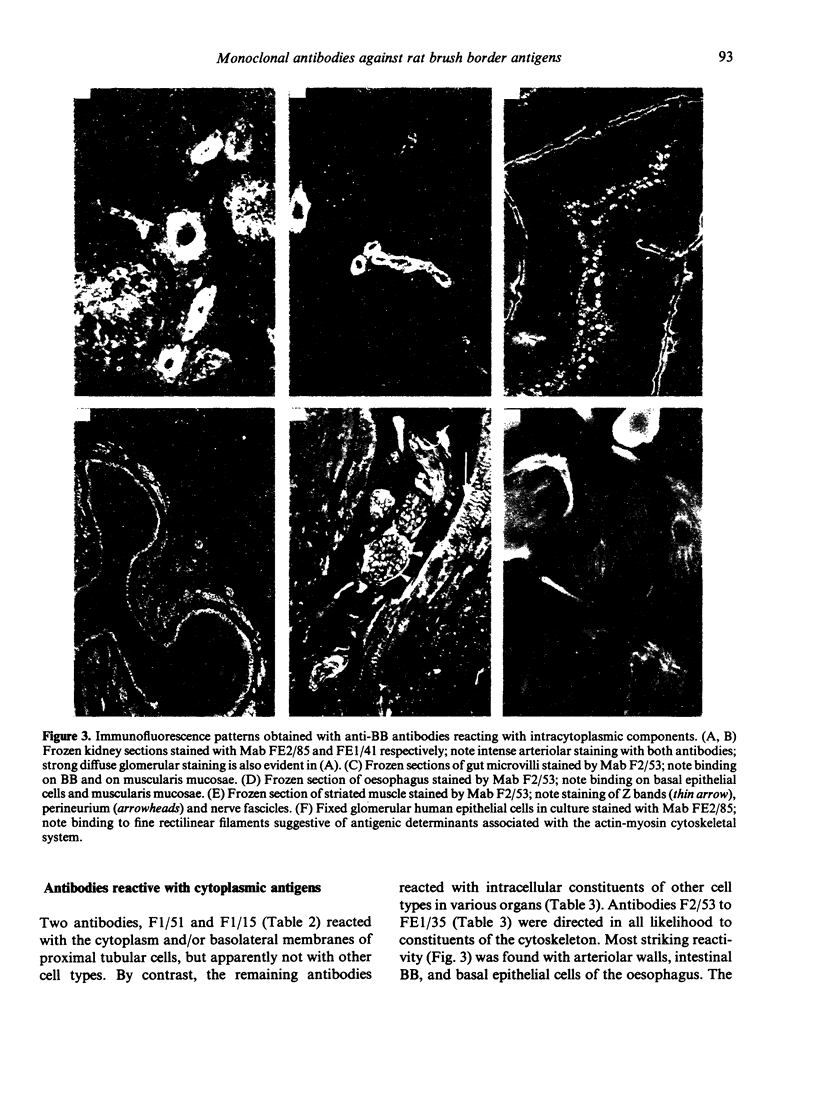


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrass C. K., McVay J., Glassock R. J. Evaluation of homologous and isologous passive Heymann nephritis: influence on endogenous antibody production. J Immunol. 1983 Jan;130(1):195–202. [PubMed] [Google Scholar]
- Andrews P. M. Investigations of cytoplasmic contractile and cytoskeletal elements in the kidney glomerulus. Kidney Int. 1981 Nov;20(5):549–562. doi: 10.1038/ki.1981.176. [DOI] [PubMed] [Google Scholar]
- Barabas A. Z., Lannigan R. Induction of an autologous immune-complex glomerulonephritis in the rat by intravenous injection of heterologous anti-rat kidney tubular antibody. I. Production of chronic progressive immune-complex glomerulonephritis. Br J Exp Pathol. 1974 Feb;55(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- Bertani T., Nolin L., Foidart J., Vandewalle A., Verroust P. The effect of puromycin on subepithelial deposits induced by antibodies directed against tubular antigens: a quantitative study. Eur J Clin Invest. 1979 Dec;9(6):465–472. doi: 10.1111/j.1365-2362.1979.tb00914.x. [DOI] [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Steinmuller D. R., Stilmant M. M., Salant D. J., Lowenstein L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978 Dec;62(6):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighiero G., Guilbert B., Avrameas S. Naturally occurring antibodies against nine common antigens in humans sera. II. High incidence of monoclonal Ig exhibiting antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J Immunol. 1982 Jun;128(6):2788–2792. [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. I. Identification and isolation of the pathogenetic antigen. J Exp Med. 1968 Mar 1;127(3):555–572. doi: 10.1084/jem.127.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuren G. J., vd Lee R., Greben H. A., Van Damme B. J., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. IV. Investigations into the pathogenesis of the model. Lab Invest. 1978 Apr;38(4):496–501. [PubMed] [Google Scholar]
- Foidart J. B., Dechenne C. A., Mahieu P., Creutz C. E., de Mey J. Tissue culture of normal rat glomeruli. Isolation and morphological characterization of two homogeneous cell lines. Invest Cell Pathol. 1979 Jan-Mar;2(1):15–26. [PubMed] [Google Scholar]
- Glassock R. J., Edgington T. S., Watson J. I., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. II. The pathogenetic mechanism. J Exp Med. 1968 Mar 1;127(3):573–588. doi: 10.1084/jem.127.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- Houghton A. N., Brooks H., Cote R. J., Taormina M. C., Oettgen H. F., Old L. J. Detection of cell surface and intracellular antigens by human monoclonal antibodies. Hybrid cell lines derived from lymphocytes of patients with malignant melanoma. J Exp Med. 1983 Jul 1;158(1):53–65. doi: 10.1084/jem.158.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Naruse T., Fukasawa T., Miyakawa Y. Laboratory model of membranous glomerulonephritis in rats induced by pronase-digested homologous renal tubular epithelial antigen. Lab Invest. 1975 Aug;33(2):141–146. [PubMed] [Google Scholar]
- Neale T. J., Wilson C. B. Glomerular antigens in Heymann's nephritis: reactivity of eluted and circulating antibody. J Immunol. 1982 Jan;128(1):323–330. [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet membrane defects in Glanzmann's thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest. 1977 Sep;60(3):535–545. doi: 10.1172/JCI108805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco P., Allegri L., Melcion C., Pirotsky E., Appay M. D., Bariety J., Pontillon F., Verroust P. A monoclonal antibody to brush border and passive Heymann nephritis. Clin Exp Immunol. 1984 Feb;55(2):319–332. [PMC free article] [PubMed] [Google Scholar]
- Striker G. E., Killen P. D., Farin F. M. Human glomerular cells in vitro: isolation and characterization. Transplant Proc. 1980 Sep;12(3 Suppl 1):88–99. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme B. J., Fleuren G. J., Bakker W. W., Vernier R. L., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978 Apr;38(4):502–510. [PubMed] [Google Scholar]
- Wilfong R. F., Neville D. M., Jr The isolation of a brush border membrane fraction from rat kidney. J Biol Chem. 1970 Nov 25;245(22):6106–6112. [PubMed] [Google Scholar]