Abstract
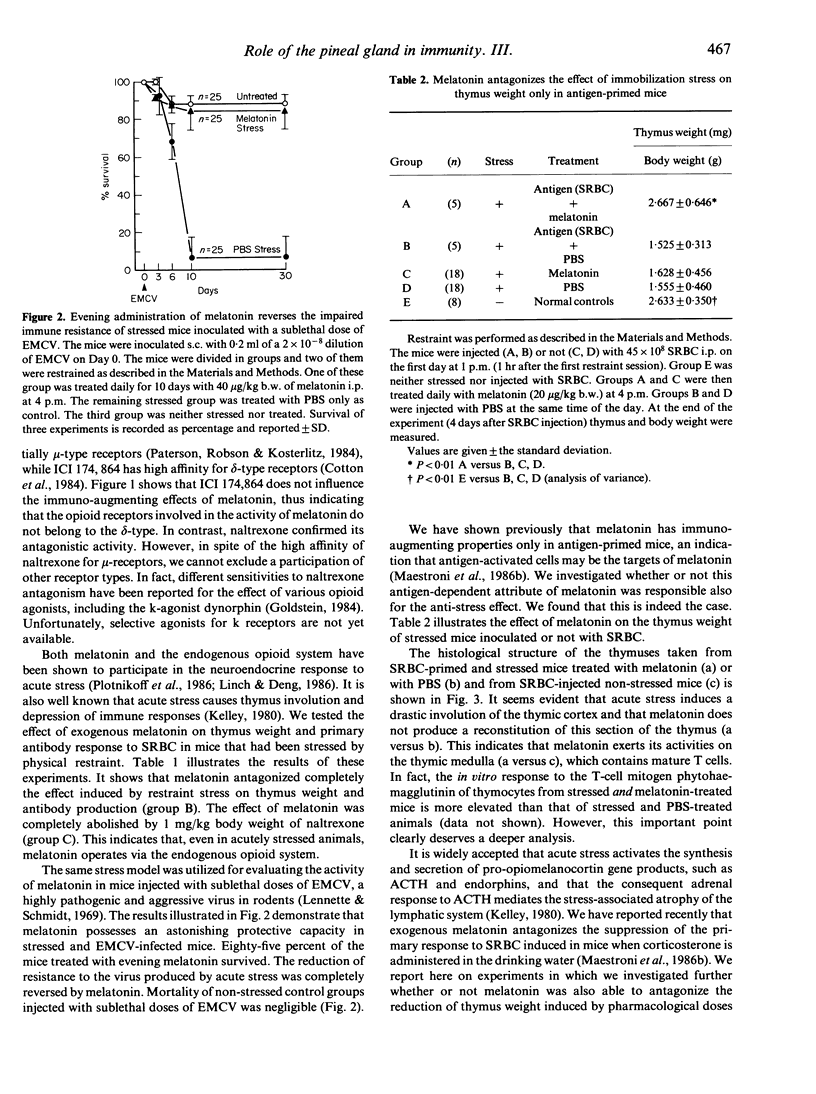
We have recently demonstrated that the pineal neurohormone melatonin exerts important immunoregulatory functions. We now report that exogenous melatonin counteracts completely the effect of acute anxiety-restraint stress on thymus weight and antibody response to sheep red blood cells (SRBC). In addition, administration of melatonin in the evening prevented paralysis and death of mice infected with sublethal doses of encephalomyocarditis virus (EMCV) after acute stress. The anti-stress activity of melatonin was present in mice injected with T-dependent antigens, and it was abolished by the contemporary administration of the specific opioid-antagonist naltrexone. This suggests that melatonin exerts its remarkable anti-stress effect on antigen-activated cells via an opiatergic mechanism. These findings have important implications at both basic and clinical levels. They provide a new approach to a possible physiological 'up-regulation' of the immune response under virus- and/or stress-related immunosuppression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besedovsky H., del Rey A., Sorkin E., Dinarello C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Cotton R., Giles M. G., Miller L., Shaw J. S., Timms D. ICI 174864: a highly selective antagonist for the opioid delta-receptor. Eur J Pharmacol. 1984 Jan 27;97(3-4):331–332. doi: 10.1016/0014-2999(84)90470-9. [DOI] [PubMed] [Google Scholar]
- Geenen V., Legros J. J., Franchimont P., Defresne M. P., Boniver J., Ivell R., Richter D. The thymus as a neuroendocrine organ. Synthesis of vasopressin and oxytocin in human thymic epithelium. Ann N Y Acad Sci. 1987;496:56–66. doi: 10.1111/j.1749-6632.1987.tb35746.x. [DOI] [PubMed] [Google Scholar]
- Kelley K. W. Stress and immune function: a bibliographic review. Ann Rech Vet. 1980;11(4):445–478. [PubMed] [Google Scholar]
- Lynch H. J., Eng J. P., Wurtman R. J. Control of pineal indole biosynthesis by changes in sympathetic tone caused by factors other than environmental lighting. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1704–1707. doi: 10.1073/pnas.70.6.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A., Pierpaoli W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J Neuroimmunol. 1986 Nov;13(1):19–30. doi: 10.1016/0165-5728(86)90047-0. [DOI] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A., Pierpaoli W. Role of the pineal gland in immunity: II. Melatonin enhances the antibody response via an opiatergic mechanism. Clin Exp Immunol. 1987 May;68(2):384–391. [PMC free article] [PubMed] [Google Scholar]



