Abstract
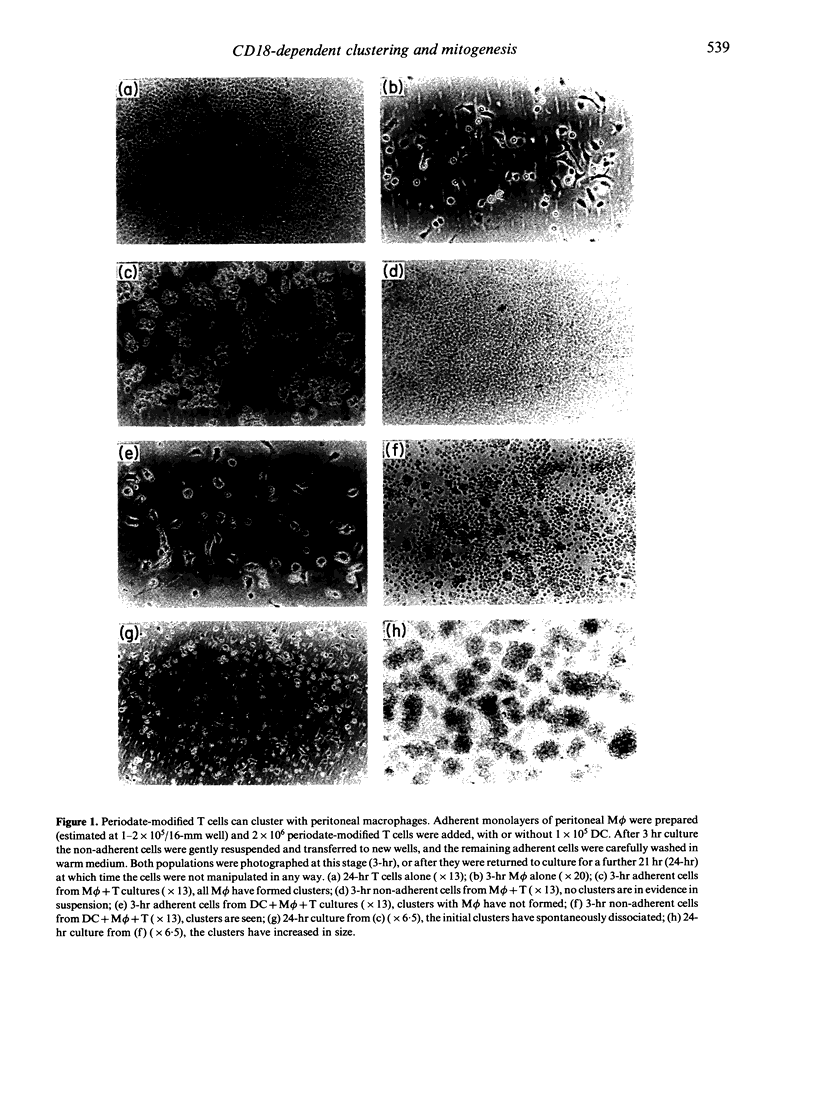
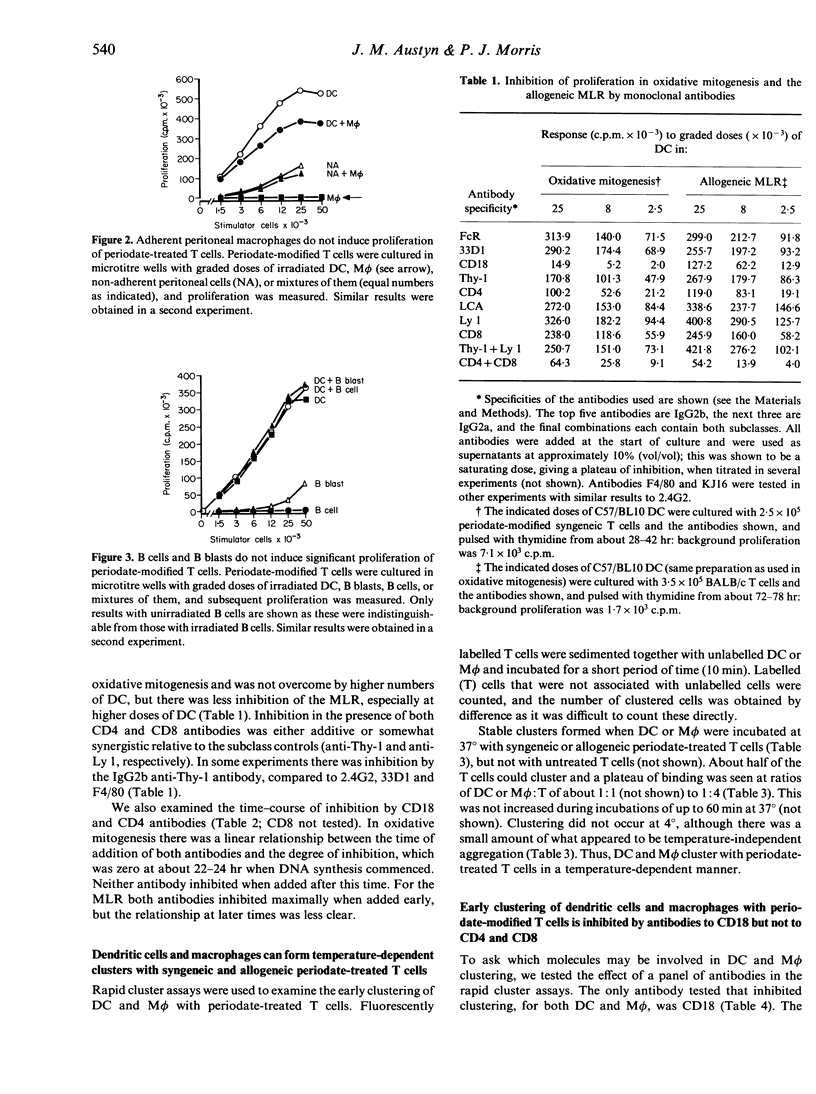
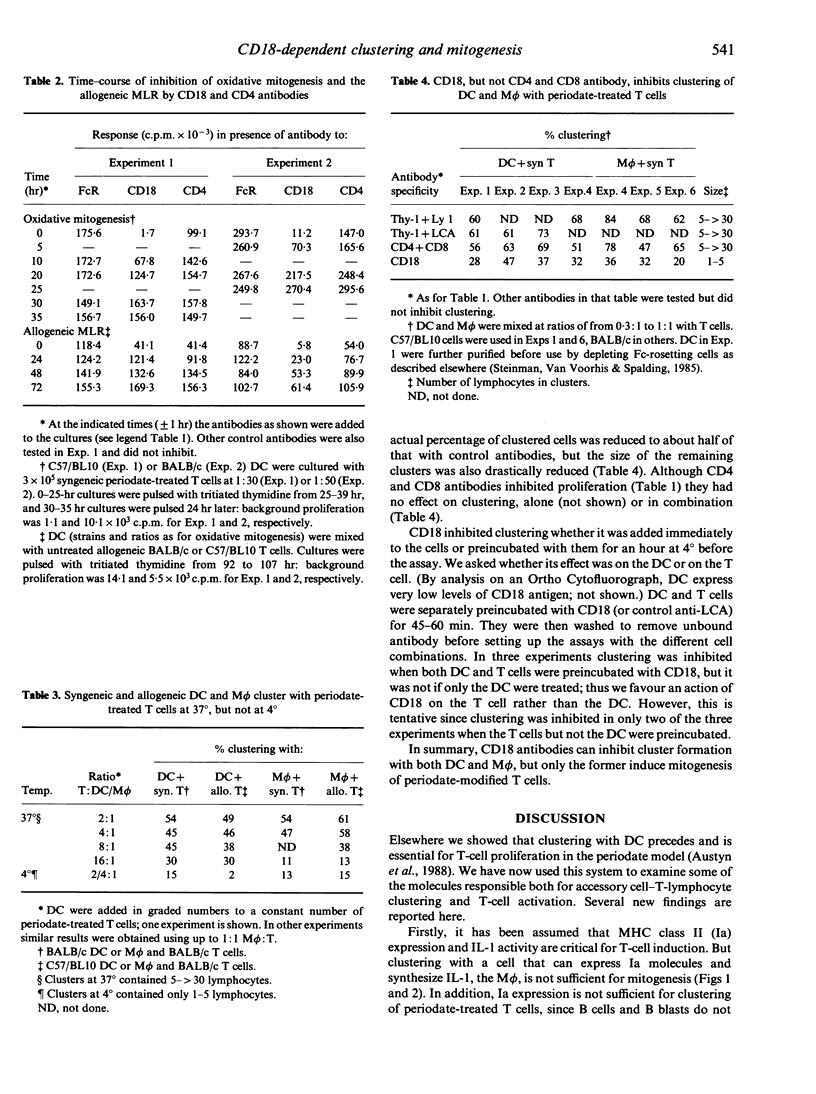
The physical association of dendritic cells and T lymphocytes in clusters is required for primary immune responses. We have used oxidative mitogenesis as a model to examine the requirements for accessory cell-T-cell clustering and T-cell activation; in this polyclonal response, periodate-treated T cells cluster with dendritic cells (DC) and proliferate. Here we observed firstly that macrophages (M phi) but not B cells or B blasts could also cluster with periodate-treated T cells, but they did not stimulate proliferation. Thus, clustering with a cell that can express MHC class II (Ia) molecules and synthesize interleukin-1 (IL-1) is not sufficient for mitogenesis, and Ia expression is not sufficient for clustering. Secondly, that proliferation in oxidative mitogenesis and the allogeneic mixed leucocyte reaction (MLR) was inhibited by CD18, CD4 and CD8 antibodies but not, with one exception, by others of the panel tested. Thirdly, using rapid cluster assays we showed that DC and M phi formed temperature-dependent clusters with syngeneic and allogeneic periodate-treated T cells. Clustering was inhibited by CD18 antibodies, probably at the level of the T cell but not the accessory cell, and this may be a general feature of such inhibition. However, CD4 and/or CD8 antibodies did not affect clustering, showing that these molecules are involved in subsequent stages of T-cell activation. Since clustering of DC and M phi with periodate-treated T cells requires CD18, but only the former leads to proliferation, we conclude that CD18-dependent clustering is not sufficient for mitogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Austyn J. M. Lymphoid dendritic cells. Immunology. 1987 Oct;62(2):161–170. [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M., Smith K. G., Morris P. J. T cell activation by anti-CD3 antibodies: function of Fc receptors on B cell blasts, but not resting B cells, and CD18 on the responding T cells. Eur J Immunol. 1987 Sep;17(9):1329–1335. doi: 10.1002/eji.1830170917. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Steinman R. M., Weinstein D. E., Granelli-Piperno A., Palladino M. A. Dendritic cells initiate a two-stage mechanism for T lymphocyte proliferation. J Exp Med. 1983 Apr 1;157(4):1101–1115. doi: 10.1084/jem.157.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenan M., Parish C. R. Intracellular fluorescent labelling of cells for analysis of lymphocyte migration. J Immunol Methods. 1984 Nov 16;74(1):31–38. doi: 10.1016/0022-1759(84)90364-8. [DOI] [PubMed] [Google Scholar]
- Green J., Jotte R. Interactions between T helper cells and dendritic cells during the rat mixed lymphocyte reaction. J Exp Med. 1985 Nov 1;162(5):1546–1560. doi: 10.1084/jem.162.5.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greineder D. K., Rosenthal A. S. The requirement for macrophage-lymphocyte interaction in T lymphocyte proliferation induced by generation of aldehydes on cell membranes. J Immunol. 1975 Oct;115(4):932–938. [PubMed] [Google Scholar]
- Gunter K. C., Kroczek R. A., Shevach E. M., Germain R. N. Functional expression of the murine Thy-1.2 gene in transfected human T cells. J Exp Med. 1986 Feb 1;163(2):285–300. doi: 10.1084/jem.163.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A., Jablonski-Westrich D., Thiele H. G. Contact interaction between lymphocytes is a general event following activation and is mediated by LFA-1. Eur J Immunol. 1986 Jul;16(7):847–850. doi: 10.1002/eji.1830160721. [DOI] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Accessory cell-T lymphocyte interactions. Antigen-dependent and -independent clustering. J Exp Med. 1986 Feb 1;163(2):247–261. doi: 10.1084/jem.163.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Monoclonal antibodies to LFA-1 and to CD4 inhibit the mixed leukocyte reaction after the antigen-dependent clustering of dendritic cells and T lymphocytes. J Exp Med. 1987 May 1;165(5):1403–1417. doi: 10.1084/jem.165.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren Z., Berke G. Interaction of periodate-oxidized target cells and cytolytic T lymphocytes: a model system of "polyclonal MHC recognition". Eur J Immunol. 1986 Sep;16(9):1049–1056. doi: 10.1002/eji.1830160904. [DOI] [PubMed] [Google Scholar]
- Koide S. L., Inaba K., Steinman R. M. Interleukin 1 enhances T-dependent immune responses by amplifying the function of dendritic cells. J Exp Med. 1987 Feb 1;165(2):515–530. doi: 10.1084/jem.165.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide S., Steinman R. M. Induction of murine interleukin 1: stimuli and responsive primary cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3802–3806. doi: 10.1073/pnas.84.11.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Witmer M. D., Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):161–165. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Hill S. W., Parker J. W., O'Brien R. L., Frelinger J. A. Characterization of responding cells in oxidative mitogen stimulation. III. Presence of I-A- and I-J, E, C-subregion gene products on the surface of required cells. Immunogenetics. 1980;10(2):133–140. doi: 10.1007/BF01561562. [DOI] [PubMed] [Google Scholar]
- Roehm N., Herron L., Cambier J., DiGuisto D., Haskins K., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells: distribution on thymus and peripheral T cells. Cell. 1984 Sep;38(2):577–584. doi: 10.1016/0092-8674(84)90512-9. [DOI] [PubMed] [Google Scholar]
- Smith K. G., Austyn J. M., Hariri G., Beverley P. C., Morris P. J. T cell activation by anti-T3 antibodies: comparison of IgG1 and IgG2b switch variants and direct evidence for accessory function of macrophage Fc receptors. Eur J Immunol. 1986 May;16(5):478–486. doi: 10.1002/eji.1830160503. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Nogueira N., Witmer M. D., Tydings J. D., Mellman I. S. Lymphokine enhances the expression and synthesis of Ia antigens on cultured mouse peritoneal macrophages. J Exp Med. 1980 Nov 1;152(5):1248–1261. doi: 10.1084/jem.152.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]



