Abstract
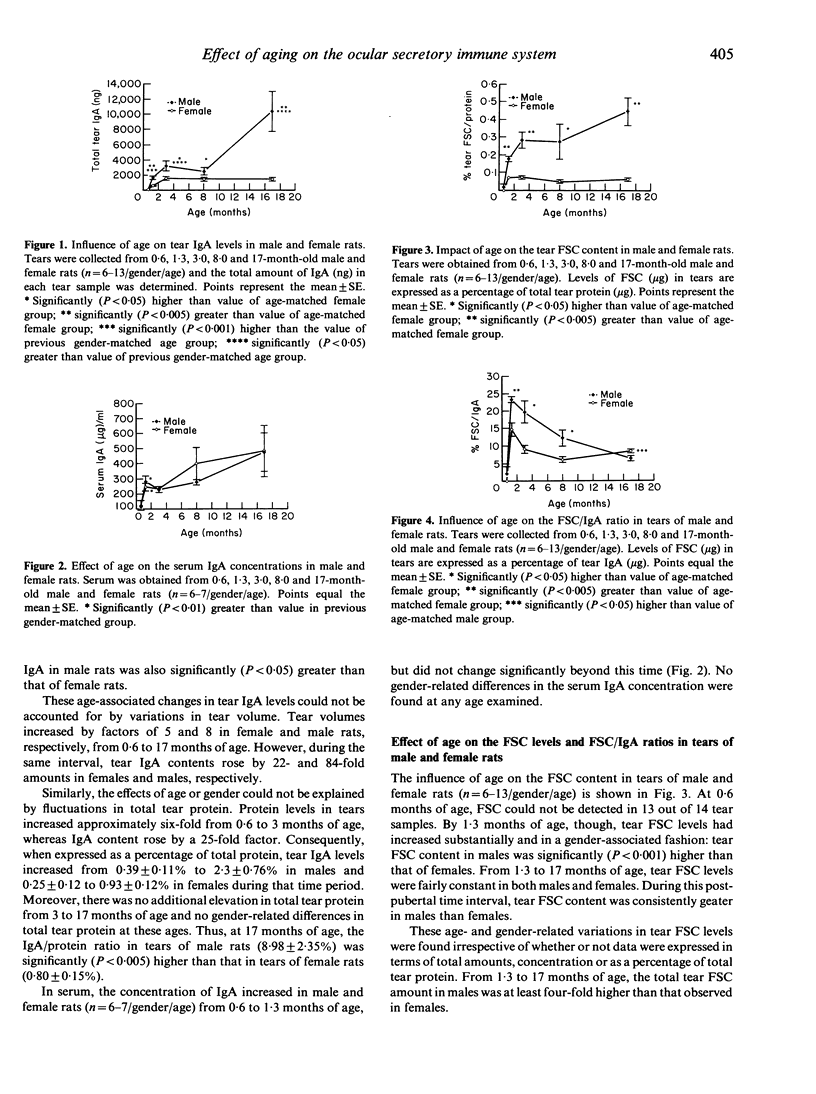
The objective of the present study was to examine the influence of aging on the ocular secretory immune system of the eye. Levels of IgA and free secretory component (FSC) were measured in lacrimal glands and/or tears of 0.6, 1.3, 3, 8 and 17-month-old male and female rats. In addition, the FSC output of lacrimal tissue cultured in vitro was evaluated. During the period from 0.6 to 1.3 months of age, the content of tear IgA increased nine- and 13-fold in females and males, respectively. This rise was paralleled by changes in the concentration of tear FSC. Prior to the onset of puberty, FSC could be detected in only 7% of tear samples, whereas after pubertal maturation, tear FSC levels had attained adult concentrations. This tear FSC profile was similar to the age-related pattern of FSC output by lacrimal tissue incubated in vitro. Following puberty, tear IgA content continued to increase in both sexes until adulthood (3 months of age) and then plateaued in females from 8 to 17 months of age. In contrast, tear IgA in males appeared to stabilize from 3 to 8 months and then rose significantly to the highest levels at 17 months of age. This increase in males was also reflected in their lacrimal tissue: IgA content underwent a six-fold elevation from 3 to 17 months. Of interest is that the differential kinetics involved in tear IgA and FSC expression resulted in an age-associated decline in the FSC/IgA ratio from post-puberty to senescence. A striking finding in these studies was the persistence of a sexual dimorphism in the secretory immune system of the eye. After pubertal development, IgA and FSC levels were significantly higher in tears of males, compared to those of females, at all ages tested up to 17 months. These gender- and age-related variations in tear IgA and FSC amounts could not be accounted for by changes in either the volume of, or total protein content in, tears.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H. Effects of chronic bronchopulmonary disease and aging on human nasal secretion IgA concentrations. J Immunol. 1968 Nov;101(5):984–988. [PubMed] [Google Scholar]
- Allansmith M. R., Gillette T. E. Secretory component in human ocular tissues. Am J Ophthalmol. 1980 Mar;89(3):353–361. doi: 10.1016/0002-9394(80)90004-5. [DOI] [PubMed] [Google Scholar]
- Allansmith M. R., Kajiyama G., Abelson M. B., Simon M. A. Plasma cell content of main and accessory lacrimal glands and conjunctiva. Am J Ophthalmol. 1976 Dec;82(6):819–826. doi: 10.1016/0002-9394(76)90056-8. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Role of J chain and secretory component in receptor-mediated glandular and hepatic transport of immunoglobulins in man. Scand J Immunol. 1985 Aug;22(2):111–146. doi: 10.1111/j.1365-3083.1985.tb01866.x. [DOI] [PubMed] [Google Scholar]
- Buts J. P., Delacroix D. L. Ontogenic changes in secretory component expression by villous and crypt cells of rat small intestine. Immunology. 1985 Jan;54(1):181–187. [PMC free article] [PubMed] [Google Scholar]
- Cavallero C. Relative effectiveness of varous steroids in an androgen assay using the exorbital lacrimal gland of the castrated rat. I. Delta-4-3-ketones and delta-5-3-beta-hydroxysteroids. Acta Endocrinol (Copenh) 1967 May;55(1):119–130. [PubMed] [Google Scholar]
- Ebersole J. L., Smith D. J., Taubman M. A. Secretory immune responses in ageing rats. I. Immunoglobulin levels. Immunology. 1985 Oct;56(2):345–350. [PMC free article] [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J. Cellular and humoral IgA responses after single and multiple local injections of antigen. Cell Immunol. 1983 Apr 15;77(2):372–384. doi: 10.1016/0008-8749(83)90038-2. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J. The effect of neonatal thymectomy on the level of salivary and serum immunoglobulins in rats. Immunology. 1979 Apr;36(4):649–657. [PMC free article] [PubMed] [Google Scholar]
- Ebling F. J., Ebling E., Randall V., Skinner J. The effects of hypophysectomy and of bovine growth hormone on the responses to testosterone of prostate, preputial, Harderian and lachrymal glands and of brown adipose tissue in the rat. J Endocrinol. 1975 Sep;66(3):401–406. doi: 10.1677/joe.0.0660401. [DOI] [PubMed] [Google Scholar]
- Gudmundsson O. G., Sullivan D. A., Bloch K. J., Allansmith M. R. The ocular secretory immune system of the rat. Exp Eye Res. 1985 Feb;40(2):231–238. doi: 10.1016/0014-4835(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Haaijman J. J., Hijmans W. Influence of age on the immunological activity and capacity of the CBA mouse. Mech Ageing Dev. 1978 May;7(5):375–398. doi: 10.1016/0047-6374(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Hahn J. D. Effect of cyproterone acetate on sexual dimorphism of the exorbital lacrimal gland in rats. J Endocrinol. 1969 Nov;45(3):421–424. doi: 10.1677/joe.0.0450421. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Jahn R., Padel U., Porsch P. H., Söling H. D. Adrenocorticotropic hormone and alpha-melanocyte-stimulating hormone induce secretion and protein phosphorylation in the rat lacrimal gland by activation of a cAMP-dependent pathway. Eur J Biochem. 1982 Sep 1;126(3):623–629. doi: 10.1111/j.1432-1033.1982.tb06826.x. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Asahina T., Kobayashi N. Differential quantification of SIgA and SC by two-directional rocket method. Immunology. 1980 Aug;40(4):597–604. [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Messiha N., Watson R. R. Immune components of the intestinal mucosae of ageing and protein deficient mice. Immunology. 1981 Jul;43(3):401–407. [PMC free article] [PubMed] [Google Scholar]
- Makinodan T., Kay M. M. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Ayyildiz A., Lemaitre-Coelho I. M., Vaerman J. P., Rockey J. H. Induction and expression of antibodies in secretions: the ocular immune system. Ann N Y Acad Sci. 1983 Jun 30;409:428–440. doi: 10.1111/j.1749-6632.1983.tb26887.x. [DOI] [PubMed] [Google Scholar]
- Nagura H., Nakane P. K., Brown W. R. Breast milk IgA binds to jejunal epithelium in suckling rats. J Immunol. 1978 Apr;120(4):1333–1339. [PubMed] [Google Scholar]
- Ramaley J. A. Development of Gonadotropin regulation in the prepubertal mammal. Biol Reprod. 1979 Feb;20(1):1–31. doi: 10.1093/biolreprod/20.1.1. [DOI] [PubMed] [Google Scholar]
- Roth G. S. Hormone action during aging: alterations and mechanisms. Mech Ageing Dev. 1979 Mar;9(5-6):497–514. doi: 10.1016/0047-6374(79)90090-3. [DOI] [PubMed] [Google Scholar]
- Schmucker D. L., Gilbert R., Jones A. L., Hradek G. T., Bazin H. Effect of aging on the hepatobiliary transport of dimeric immunoglobulin A in the male Fischer rat. Gastroenterology. 1985 Feb;88(2):436–443. doi: 10.1016/0016-5085(85)90504-9. [DOI] [PubMed] [Google Scholar]
- Shaw P. H., Held W. A., Hastie N. D. The gene family for major urinary proteins: expression in several secretory tissues of the mouse. Cell. 1983 Mar;32(3):755–761. doi: 10.1016/0092-8674(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Ebersole J. L., Taubman M. A. Local and systemic immune response in aged hamsters. Immunology. 1983 Nov;50(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- Steiner R. A., Bremner W. J., Clifton D. K., Dorsa D. M. Reduced pulsatile luteinizing hormone and testosterone secretion with aging in the male rat. Biol Reprod. 1984 Sep;31(2):251–258. doi: 10.1095/biolreprod31.2.251. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Allansmith M. R. Hormonal influence on the secretory immune system of the eye: androgen modulation of IgA levels in tears of rats. J Immunol. 1985 May;134(5):2978–2982. [PubMed] [Google Scholar]
- Sullivan D. A., Allansmith M. R. Hormonal influence on the secretory immune system of the eye: endocrine interactions in the control of IgA and secretory component levels in tears of rats. Immunology. 1987 Mar;60(3):337–343. [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Allansmith M. R. Hormonal modulation of tear volume in the rat. Exp Eye Res. 1986 Feb;42(2):131–139. doi: 10.1016/0014-4835(86)90037-0. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Allansmith M. R. Source of IgA in tears of rats. Immunology. 1984 Dec;53(4):791–799. [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Bloch K. J., Allansmith M. R. Hormonal influence on the secretory immune system of the eye: androgen control of secretory component production by the rat exorbital gland. Immunology. 1984 Jun;52(2):239–246. [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Bloch K. J., Allansmith M. R. Hormonal influence on the secretory immune system of the eye: androgen regulation of secretory component levels in rat tears. J Immunol. 1984 Mar;132(3):1130–1135. [PubMed] [Google Scholar]
- Sullivan D. A., Colby E. B., Hann L. E., Allansmith M. R., Wira C. R. Production and utilization of a mouse monoclonal antibody to rat IgA: identification of gender-related differences in the secretory immune system. Immunol Invest. 1986 Jun;15(4):311–325. doi: 10.3109/08820138609052950. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Wira C. R. Variations in free secretory component levels in mucosal secretions of the rat. J Immunol. 1983 Mar;130(3):1330–1335. [PubMed] [Google Scholar]
- Wade A. W., Szewczuk M. R. Aging, idiotype repertoire shifts, and compartmentalization of the mucosal-associated lymphoid system. Adv Immunol. 1984;36:143–188. doi: 10.1016/s0065-2776(08)60901-3. [DOI] [PubMed] [Google Scholar]


