Introduction
Eukaryotic plant cells contain different organelles, among them mitochondria and plastids. Chloroplasts and mitochondria have most probably arisen from two independent endosymbiotic events—mitochondria from proteobacterial ancestors, and chloroplasts from ancient cyanobacteria.
Mitochondria function as power ‘houses’, supplying aerobic cells with energy in the form of ATP. To maintain their functionality it is essential that solutes and metabolites pass through the two membranes that enclose mitochondria. The inner membrane contains numerous α-helical transporter proteins, whereas in the outer membrane only two β-barrel pore-forming proteins have been identified so far. They represent isoforms of the mitochondrial porin called VDAC (voltage-dependent anion channel). Plant plastids perform a number of vital biochemical functions including photosynthetic CO2 fixation, fatty acid biosynthesis, aromatic, branched chain and hydroxylated amino acid biosynthesis, porphyrin biosynthesis, and sulfate and nitrate reduction. These biosynthetic activities are regulated by environmental factors such as light, temperature and nutrient availability. The metabolism of the entire plant is mediated by an ensemble of metabolic ‘switches’, which redirect the physiology of plastids. Chloroplasts continuously exchange ions, metabolic intermediates and photosynthetic products with the cytosol over the barriers of the inner and outer envelope membranes. In contrast to mitochondria, several ion channels showing different properties have been characterized in the outer envelope. This review will argue that the current assumption that solute movement across the outer organellar membranes is determined solely by size limits is questionable and possibly an oversimplification. Due to their evolutionary history, both types of organelle share certain features that are related to both of the different bacterial ancestors. By analogy to Gram-negative bacteria, which represent the evolutionary progenitors of plastids and mitochondria (Margulis, 1970; Gray, 1993), the intermembrane space is topologically equivalent to the periplasm, a compartment characterized by distinct biochemical functions. Consequently, the intermembrane space should not be envisaged simply as a continuum of the cytosol, but rather as a ‘buffer zone’ between the organelle and the cytosol. To enhance this buffering capability, permeability of the outer membrane/envelope must be regulated to respond to changes in the metabolic needs of the organelle.
We will, therefore, compare the feature of solute transport across the outer membranes of bacteria with what is known about the translocation processes across the organellar outer membranes. In particular, we will explore novel regulatory mechanisms, which may be absent in prokaryotes, that enhance the functionality of these ion channels and incorporate them intimately into the cell physiology of eukaryotes.
Bacterial porins
Porins are defined as water-filled channels through which molecules of up to 600 Da may diffuse (Nikaido and Rosenberg, 1981). In contrast to permeases and transport proteins located in the inner membrane, in which transmembrane domains are formed by four or more hydrophobic α-helices, porins are composed of transmembrane amphipathic β-strands and are exclusively located in the outer membrane. These β-strands form β-barrel structures that constitute the actual pore. The membrane-spanning β-strands are connected by hydrophilic loops, extending either into the intermembrane space or into the extracellular environment. These loops play an important role in the ion selectivity and regulation of the channels. In Gram-negative bacteria, porins mediate the uptake of a wide range of nutrients across the outer membrane.
Gram-negative bacteria contain three different classes of porin (Klebba and Newton, 1998): (i) general porins, exemplified by OmpF, OmpC and PhoE, which do not bind solutes; (ii) solute-specific porins like LamB, which bind their solutes with relatively low affinity; and (iii) ligand-gated, energy-dependent porins like FepA or FhuB, characterized by high-affinity binding to their specific ligand.
Crystal structure analyses revealed β-barrel structure for all three classes, although the number of pore-forming β-strands is different. General porins contain 16, specific porins 18 (for review see Nikaido, 1993) and FepA porins 22 putative membrane-spanning β-strands (Ferguson et al., 1998; Locher et al., 1998).
Most outer membrane porins are functional as trimeric homo-oligomers. The external loops form an extensive vestibule in which candidate solutes are gathered. The third loop (L3) of general porins folds into the channel and thereby constricts the diameter of the aqueous channel to 7 × 11 Å (Nikaido and Saier, 1992), resulting in an exclusion limit of ∼600 Da. Furthermore, the strategic placement of negatively charged amino acids in L3 of OmpC and OmpF is proposed to be responsible for their slight cation selectivity. Conversely, PhoE contains an additional lysine residue within the L3 region and, therefore, preferentially transports anions (Samartzidou and Delcour, 1998).
Collectively, the general diffusion porins have been considered to rest in an open state (Berrier et al., 1992). Support for this concept has come from in vitro measurements using reconstituted proteins that form open channels in black lipid bilayer membranes at a membrane potential of 0 mV, but close at potentials higher than 100 mV (Schindler and Rosenbusch, 1978; Dargent et al., 1986). However, no membrane potential across the outer membrane has been observed, except a Donnan potential of ∼30 mV (Sen et al., 1988). Moreover, this effect of only 30 mV is much lower than the ‘closing potential’ of 100 mV observed for the general porins in vitro.
Thus, it seems unlikely that the gating observed in vivo is controlled via a purely voltage-dependent mechanism. Although the mechanics of channel gating are still elusive, it is apparent that one or more levels of regulation must exist (Klebba and Newton, 1998). Interesting experiments in this field have been executed by Delcour and collegues (DelaVega and Delcour, 1996; Iyer and Delcour, 1997; Samartzidou and Delcour, 1999). They examined the influence of polycations like polyamines on the channel gating of OmpF, OmpC and PhoE. Although highly homologous to each other, each of these porins exhibited distinct sensitivity to modulation by polyamines. Moreover, these compounds have been shown to bind to at least two distinct binding sites, one of which resides within the pore (Iyer and Delcour, 1997).
Polyamines are naturally occurring molecules that associate tightly with the negatively charged lipopolysaccharide layer of Gram-negative bacteria (Koski and Vaara, 1991). In eukaryotes, they have been shown to modulate ion channels of heart, muscle and neurons (Scott et al., 1993; Ficker et al., 1994; Lopatin et al., 1994; Bowie and Mayer, 1995; Gomez and Hellstrand, 1995; Johnson, 1996; Uehara et al., 1996), indicating that polyamines may function in a novel way to regulate channel gating in both prokaryotes and eukaryotes.
Expression of the genes encoding the different porins is also highly regulated: in addition to substrate regulation, expression of OmpC is stimulated under nitrogen limitation, whereas OmpF expression rises upon glucose limitation (Liu and Ferenci, 1998). PhoE transcription is induced during phosphate-limited growth conditions (Tommassen et al., 1982).
Solute-specific porins
The maltoporin LamB is characterized by low-affinity binding of maltodextrins. Although LamB resembles the general porins, periplasmic access to its aqueous channel is shielded by the extra-membranous loops L4, L6 and L9, which form an umbrella-like structure above the pore entrance (Klebba et al., 1994). Furthermore, binding of maltodextrins also involves residues in L9 (Klebba et al., 1997). Although the exact pathway is not clear, movement of maltodextrin through the channel may involve a cascade of specifically orientated aromatic residues that collectively function as a ‘greasy slide’.
A completely different type of porin is represented by the ferric enterobactin transporter, FepA. Although FepA has a similar pore-forming β-barrel structure and extending surface loops, the transport mechanism is fundamentally different to other porins. The pore is generally closed by surface loops that create a high-affinity binding site for iron chelated by enterobactin (Newton et al., 1997). The transport process is initiated by ligand binding to L5, which opens the ‘lid’, thus allowing solute entry (Newton et al., 1997). In contrast to the facilitated diffusion process mediated by OmpC or LamB, translocation of iron through FepA involves chelation by the siderophore enterobactin and is strictly energy dependent. This energy requirement involves a membrane potential and induces conformational changes within the channel. The changes in channel conformation not only occur upon ligand binding but also proceed throughout the whole transport process (Jiang et al., 1997). The energy is most probably provided via interactions with TonB, which is essential for the function of FepA (Wang and Newton, 1969). Although TonB is located in the bacterial plasma membrane, the bulk of the protein extends into the periplasm and is able to contact FepA in the outer membrane (Klebba et al., 1993). This contact somehow transduces the energy status of the plasma membrane, inducing the conformational changes seen in FepA. Although the exact mechanism of TonB action remains unclear, a number of studies indicate that TonB acts as a transducer deploying energy from the membrane potential across the plasma membrane to FepA located in the outer membrane (Skare et al., 1993; Larsen et al., 1994, 1997).
In conclusion, solute transport across bacterial outer membranes is definitely not a passive diffusion process through unregulated ion channels, but may involve solute-mediated, regulated and energy-dependent processes.
Mitochondrial porin
The mitochondrial porin in the outer membrane—also called VDAC—has been characterized as a relatively non-specific general diffusion pore (for review see Mannella, 1997). It shares general structural and functional properties with bacterial general porins OmpC and OmpF. It is thought to form a homodimer, with each 30 kDa subunit (Thomas et al., 1991) consisting of 16 antiparallel amphipathic β-strands (Benz, 1994), which form the actual pore. Membrane potentials >30 mV lead to the channel having a lower conductance ‘closed’ state, which is slightly cation selective (Benz et al., 1990), and no longer permeable to nucleoside triphosphates (Rostovtseva and Colombini, 1996). It was widely held that VDAC was normally in the open state, considering the absence of a membrane potential across the mitochondrial outer membrane. However, in recent years evidence has emerged that VDAC is, in fact, normally closed and highly regulated by metabolites, substrates and nucleotides, which control the permeability of the outer membrane. Several groups have shown that the permeability of mitochondrial porins is strongly influenced by modulator proteins located in the intermembrane space (Holden and Colombini, 1988; Liu and Colombini, 1991, 1992), or by β-NADH (Lee et al., 1994). This permits metabolites such as NADH to control nucleotide exchange across the outer membrane via VDAC, thereby exerting control on respiration (Liu and Colombini, 1992; Lee et al., 1994). One model predicts that VDAC modulator proteins function by increasing the gating sensitivity of the VDAC channels, thereby switching the channel to the low-conducting closed-state conformation (Liu and Colombini, 1991). The regulatory function of these modulators seems conserved in eukaryotes, indicating a wide-spread physiological importance. The biochemical nature of this modulator is still to be investigated and the results may provide new insights into both mitochondrial function and regulation.
Another essential role of VDAC has been demonstrated by Shimizu et al. (2000), implicating VDAC as a critical factor in apoptosis. The disturbed ATP/ADP exchange mediated by VDAC leads to further apoptotic events, including the release of cytochrome c into the cytosol potentially through VDAC (Vander Heiden et al., 2000).
Further evidence of VDAC regulation by nucleotides comes from the analysis of the bacterial porin PorB from Neisseria species, a clinically relevant pathogen that releases a pore-forming toxin causing disease in humans. Rudel and co-workers (Rudel et al., 1996) elegantly demonstrated that the gating of PorB is regulated by nucleotide binding to the specific amino acid triplet GLK. Mutations of the GLK triplet result in the loss of nucleotide binding and nucleotide-dependent channel gating. Although no sequence similarity between PorB and VDACs from different organisms is evident (≤30%) (Benz, 1994), the conserved motif containing GLK occurs in all of them. It is possible that not only the permeability for nucleotides is influenced by modulators or β-NADH but also nucleotides themselves may regulate the open state and conducting properties of mitochondrial porins. Thus far, no direct regulation of VDAC by nucleotide binding has been demonstrated.1
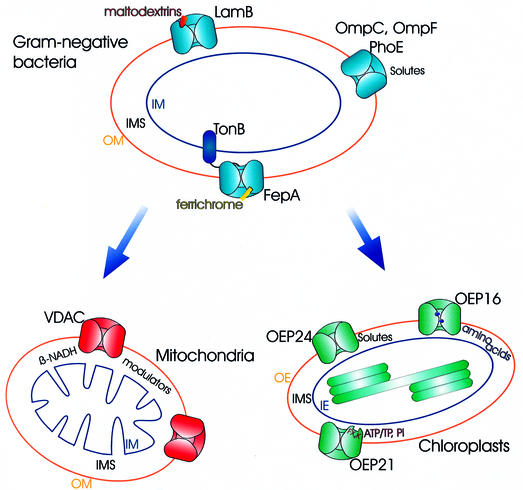
Fig. 1. Working model of solute transport across outer membranes. The model indicates the functional diversity and continuity of bacterial outer membrane channels with those in the outer membrane of chloroplasts and mitochondria. The scheme is not drawn to scale nor can it represent the complexity of the situation in vivo.
Chloroplast porins
Chloroplasts are like Gram-negative bacteria and mitochondria in that they are surrounded by two membranes. In chloroplasts the inner envelope membrane has been considered to be the actual permeability barrier (Flügge and Benz, 1984), whereas the outer envelope has been assumed to be permeable to low molecular weight solutes (<600 Da) due to the presence of porins (Flügge and Benz, 1984; Pottosin, 1992; Heiber et al., 1995). This led to a notion that the outer membrane is a simple size-selective sieve. However, three pore-forming proteins (OEP16, OEP21 and OEP24) in the outer envelope have recently been characterized (Pohlmeyer et al., 1997, 1998; Bölter et al., 1999), each exhibiting differences in substrate specificity and gating. Secondary structure predictions suggest that OEP21 and OEP24 have a β-barrel-like structure containing eight and seven membrane-span ning β-strands, respectively. OEP16 is unusual in that it is predicted to have a mixed structure, with four N-terminal β-strands plus three C-terminal α-helices. Although the β-barrel structure of these OEPs is reminiscent of bacterial porins and VDAC, they clearly differ in the number of β-strands. In this aspect they resemble a small family of bacterial porins, namely OmpA, OprF and Omp21, which also contain β-strands and are functional as monomers (Nikaido et al., 1991; Sugawara and Nikaido, 1992; Baldermann et al., 1998). Biochemical results suggest that all three chloroplastic channels form homo-oligomers (Pohlmeyer et al., 1997, 1998; Bölter et al., 1999). All three proteins have been heterologously expressed and reconstituted into artificial bilayers for electrophysiological measurements. They represent high-conductance solute channels, with the highest open probability at 0 mV and pronounced closing at increasing membrane potentials. Their distinct substrate specificities indicate separate roles in different metabolic processes, challenging the notion that they are general diffusion pores. OEP16 forms a high-conductance channel (Pohlmeyer et al., 1997) with selective permeability to amino acids and compounds with primary amino groups. Although the estimated pore diameter of 0.8–1.0 nm would be sufficiently large to pass different solutes, uncharged sugars or negatively charged carbohydrates like 3-phosphoglycerate are somehow excluded. Thus, OEP16 resembles LamB in its selectivity characteristics and is definitely not functioning as a general diffusion pore.
Recent experiments have identified the actual pore-forming region of OEP16 (Steinkamp et al., 2000). In contrast to the classical bacterial homotrimeric porins, where a monomeric barrel comprises 16 or 18 β-strands (for review see Klebba and Newton, 1998), the minimal OEP16 unit consists of only 71 residues (amino acids 22–93), which potentially encode two β-strands and one α-helix. OEP16 is present in plastids from leaves, roots and shoots. Plastids from these tissues function as the sole site of nitrate reduction to -NH2, and provide nitrogen in the form of glutamine or asparagine to other compartments in the cells. It is, therefore, tempting to assume a role for OEP16 in plant nitrogen metabolism.
A cold-regulated gene in barley was identified as an OEP16 homologue (Baldi et al., 1999). This gene, cor tmc-ap3, is expressed at a constant low level exclusively in leaf chloroplasts but is upregulated upon growth at low temperatures. This increase of mRNA is accompanied by an increase of the corresponding protein. In barley, the OEP16 homologue plays a role in frost tolerance. Other grain species that cannot increase OEP16 expression upon cold induction are frost intolerant. An increase in free amino acids and amines is part of cold acclimatization. The ion-channel function of OEP16 seems to serve different physiological purposes depending on the environmental conditions, while the solute selectivity remains similar. At least three different isoforms of OEP16 can be detected in the Arabidopsis genome.
In contrast to the other two outer envelope ion channels, a stretch of ∼50 amino acids of OEP16 shares sequence similarities with the mitochondrial protein translocation components Tim17, Tim22 and Tim23, as well as with the Escherichia coli amino acid permease, LivH (Rassow et al., 1999). This suggests that a common motif might exist in channel proteins present in both inner and outer organellar membranes, which originated early in evolution and has been used as a module to form different channel proteins.
OEP24, on the other hand, is more similar to the mitochondrial VDAC with respect to its low solute specificity (Pohlmeyer et al., 1998). This notion is further supported by the observation that OEP24 can functionally complement a yeast strain deficient in mitochondrial VDAC (Röhl et al., 1999). In this system, OEP24 is obviously mistargeted to the outer mitochondrial membrane, where it operates as a general diffusion pore. In vitro OEP24 gating is voltage dependent, but it is reasonable to assume that OEP24 is modulated by proteinaceous factors and/or metabolites, as could be demonstrated for VDAC (Liu and Colombini, 1992; Lee et al., 1994; also see below).
So far, the most complex ion channel of the outer envelope, with respect to its substrate specificity and gating properties, is OEP21 (Bölter et al., 1999). OEP21 constitutes an anion-selective channel with a relatively low conductance. It prefers inorganic phosphate (Pi) and phosphorylated metabolic intermediates [triosephosphates (TP), 3-phosphoglyceric acid (3-PGA)] over other anions like chloride. Unexpectedly, OEP21 has intrinsically rectifying properties. In the presence of physiological ATP levels both the reversal potential and the current– voltage characteristics change dramatically, resulting in a slightly cation-selective channel with opposite rectification direction. ATP binding causes a switch from inwardly to outwardly rectifying OEP21. The actual regulation is mediated by the ratio of ATP to TP/Pi in the intermembrane space. Low ATP:TP/Pi ratios, which occur during high photosynthetic activity, lead to outwardly rectifying OEP21 channels and thus to the transport of TP and 3-PGA into the cytosol. Under dark conditions the TP concentration in the intermembrane space decreases drastically, resulting in a high ATP:TP ratio. Consequently, OEP21 permeates metabolites from the cytosol forward to the inner envelope of chloroplasts. Simultaneously the ion selectivity decreases. Thus, it seems obvious that the outer envelope functions as a selective, regulated ‘molecular sieve’.
Changes in permeation properties upon ATP binding were first characterized in potassium-selective inwardly rectifying channels in sarcoplasmic reticulum (McIntosh et al., 1996; Drain et al., 1998). These channels, which are inhibited by ATP, consist of a hetero-octamer of four sulfonylurea receptor (SUR) and four inwardly rectifying K+ channel (Kir) subunits (McIntosh et al., 1996). SUR is a member of the ATP-binding cassette (ABC) family of proteins and acts as a regulatory subunit, conferring ADP sensitivity and the distinct pharmacological characteristics on the KATP channel complex. In contrast, the Kir subunit forms the pore of the channel and mediates the defining ATP-dependent inhibition of KATP channels. They contain an FX4K motif that is proposed to bind ATP. Site-directed mutagenesis demonstrated that both the phenylalanine and lysine residues are essential for channel function. OEP21 contains a FX4K motif at the C-terminus, which is located in the intermembrane space between the outer and the inner chloroplastic envelope. Deletion of the FX4K motif from OEP21 results in a loss of ATP regulation (R.Wagner and J.Soll, unpublished results). Furthermore, it has been shown recently that the ATP-binding properties of potassium channels are influenced by phosphorylation by protein kinase C (PKC) on a typical consensus site (Light et al., 2000). On the extreme N-terminus of OEP21 a similar phosphorylation consensus site can be found, serine at position 4 to arginine at position 6, suggesting another level of regulation.
Considering that all three chloroplastic channel proteins have the highest open probability at 0 mV, it would be reasonable to assume that reconstituted outer envelope membranes also contain these channels in the open state. However, OEP21 is the only solute channel that is detected in an open state when isolated outer membranes are fused with planar bilayers (Bölter et al., 1999). The OEP24 channel, which in vitro is also permeable to TP and anions, seems to be non-conducting under in vivo conditions. This suggests that the gating of OEP24 and OEP16 is obviously regulated by means other than the membrane potential. To maintain the regulative function of the outer envelope, it is likely that OEP21 is also normally closed in vivo, with the essential components necessary to achieve the closed state lost or inactivated during purification of envelope membranes. The conductivity states of the different channels may react to, and thereby reflect the physiological situation of the plastid—being controlled by the presence and ratio of the respective solutes (Soll et al., 2000).
Conclusions
In summary, it can be said that the black and white picture of outer membrane pores—from bacteria to mitochondria and chloroplasts—as generally open non-selective diffusion pores has to be filled with colour: outer membranes retain the potential of coarse control over metabolism by the existence of specific regulated channel-forming proteins. Although in vitro experiments on the effect of voltage changes are useful in studying ion channels, such reconstituted systems present an oversimplification of what actually occurs in the cell. However, in vivo it seems that solutes, metabolites and proteinaceous factors are responsible for channel gating—ushering or holding back solutes by pressing the communication button of the gate. A working model of transport across outer membranes is shown in Figure 1.
Acknowledgments
Acknowledgements
We would like to thank B.Bruce, G.McFadden and G.van Dooren for critical reading of the manuscript. The research was supported by grants from the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
References
- Baldermann C., Lupas,A., Lubieniecki,J. and Engelhardt,H.J. (1998) The regulated outer membrane protein Omp21 from Comamonas acidovorans is identified as a member of a new family of eight-stranded β-sheet proteins by its sequence and properties. J. Bacteriol., 180, 3741–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi P., Grossi,M., Pecchioni,N., Vale,G. and Cattivelli,L. (1999) High expression level of a gene coding for a chloroplastic amino acid selective channel protein is correlated to cold acclimation in cereals. Plant Mol. Biol., 41, 233–243. [DOI] [PubMed] [Google Scholar]
- Benz R. (1994) Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim. Biophys. Acta, 1197, 167–196. [DOI] [PubMed] [Google Scholar]
- Benz R., Kottke,M. and Brdiczka,D. (1990) The cationically selective state of the mitochondrial outer membrane pore: a study with intact mitochondria and reconstituted mitochondrial porin. Biochim. Biophys. Acta, 1022, 311–318. [DOI] [PubMed] [Google Scholar]
- Berrier C., Coulombe,A., Houssin,C. and Ghazi,A. (1992) Fast and slow kinetics of porin channels from Escherichia coli reconstituted into giant liposomes and studied by patch–clamp. FEBS Lett., 306, 251–256. [DOI] [PubMed] [Google Scholar]
- Bölter B., Soll,J., Hill,K., Hemmler,R. and Wagner,R. (1999) A rectifying ATP-regulated solute channel in the chloroplastic outer envelope from pea. EMBO J., 18, 5505–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D. and Mayer,M.L. (1995) Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron, 15, 453–462. [DOI] [PubMed] [Google Scholar]
- Dargent B., Hofmann,W., Pattus,F. and Rosenbusch,J.P. (1986) The selectivity filter of voltage-dependent channels formed by phosphoporin (PhoE) from E.coli. EMBO J., 5, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelaVega A.L. and Delcour,A.H. (1996) Polyamines decrease Escherichia coli outer membrane permeability. J. Bacteriol., 178, 3715–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain P., Li,L. and Wang,J. (1998) KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc. Natl Acad. Sci. USA, 95, 13953–13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A.D., Hofmann,E., Coulton,J.W., Diederichs,K. and Welte,W. (1998) Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science, 282, 2215–2220. [DOI] [PubMed] [Google Scholar]
- Ficker E., Taglialatea,M., Wible,B.A., Henley,C.M. and Brown,A.M. (1994) Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science, 266, 1068–1072. [DOI] [PubMed] [Google Scholar]
- Flügge U.I. and Benz,R. (1984) Pore-forming activity in the outer membrane of the chloroplast envelope. FEBS Lett., 169, 85–89. [Google Scholar]
- Gomez M. and Hellstrand,P. (1995) Endogenous polyamines modulate Ca2+ channel activity in guinea-pig intestinal smooth muscle. Pflugers Arch., 430, 501–507. [DOI] [PubMed] [Google Scholar]
- Gray M.W. (1993) Origin and evolution of organelle genomes. Curr. Opin. Genet. Dev., 3, 884–890. [DOI] [PubMed] [Google Scholar]
- Heiber T., Steinkamp,T., Hinnah,S., Schwarz,M., Flugge,U.I., Weber,A. and Wagner,R. (1995) Ion channels in the chloroplast envelope membrane. Biochemistry, 34, 15906–15917. [DOI] [PubMed] [Google Scholar]
- Holden M.J. and Colombini,M. (1988) The mitochondrial outer membrane channel, VDAC, is modulated by a soluble protein. FEBS Lett., 241, 105–109. [DOI] [PubMed] [Google Scholar]
- Iyer R. and Delcour,A.H. (1997) Complex inhibition of OmpF and OmpC bacterial porins by polyamines. J. Biol. Chem., 272, 18595–18601. [DOI] [PubMed] [Google Scholar]
- Jiang X., Payne,M.A., Cao,Z., Foster,S.B., Feix,J.B., Newton,S.M. and Klebba,P.E. (1997) Ligand-specific opening of a gated-porin channel in the outer membrane of living bacteria. Science, 276, 1261–1264. [DOI] [PubMed] [Google Scholar]
- Johnson T.D. (1996) Modulation of channel function by polyamines. Trends Pharmacol. Sci., 17, 22–27. [DOI] [PubMed] [Google Scholar]
- Klebba P.E. and Newton,S.M. (1998) Mechanisms of solute transport through outer membrane porins: burning down the house. Curr. Opin. Microbiol., 1, 238–247. [DOI] [PubMed] [Google Scholar]
- Klebba P.E., Rutz,J.M., Liu,J. and Murphy,K. (1993) Mechanisms of TonB-catalyzed iron transport through the enteric bacterial cell envelope. J. Bioenerg. Biomembr., 25, 603–611. [DOI] [PubMed] [Google Scholar]
- Klebba P.E., Hofnung,M. and Charbit,A. (1994) A model of maltodextrin transport through the sugar-specific porin, LamB, based on deletion analysis. EMBO J., 13, 4670–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebba P.E., Newton,S.M., Charbit,A., Michel,V., Perrin,D. and Hofnung,M. (1997) Further genetic analysis of the C-terminal external loop region in Escherichia coli maltoporin. Res. Microbiol., 148, 375–387. [DOI] [PubMed] [Google Scholar]
- Koski P and Vaara,M. (1991) Polyamines as constituents of the outer membranes of Escherichia coli and Salmonella typhimurium. J. Bacteriol., 173, 3695–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R.A., Thomas,M.G., Wood,G.E. and Postle,K. (1994) Partial suppression of an Escherichia coli TonB transmembrane domain mutation (ΔV17) by a missense mutation in ExbB. Mol. Microbiol., 13, 627–640. [DOI] [PubMed] [Google Scholar]
- Larsen R.A., Foster-Harnett,D., McIntosh,M.A. and Postle,K. (1997) Regions of Escherichia coli TonB and FepA proteins essential for in vivo physical interactions. J. Bacteriol., 179, 3213–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.C., Xu,X., Blachly-Dyson,E., Forte,M. and Colombini,M. (1994) β-NADH decreases the permeability of the mitochondrial outer membrane to ADP by a factor of 6. J. Biol. Chem., 269, 30974–30980. [PubMed] [Google Scholar]
- Light P.E., Bladen,C., Winkfein,R.J., Walsh,M.P. and French,R.J. (2000) Molecular basis of protein kinase C-induced activation of ATP-sensitive potassium channels. Proc. Natl Acad. Sci. USA, 97, 9058–9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.Y. and Colombini,M. (1991) Voltage gating of the mitochondrial outer membrane channel VDAC is regulated by a very conserved protein. Am. J. Physiol., 260, C371–C374. [DOI] [PubMed] [Google Scholar]
- Liu M.Y. and Colombini,M. (1992) Regulation of mitochondrial respiration by controlling the permeability of the outer membrane through the mitochondrial channel, VDAC. Biochim. Biophys. Acta, 1098, 255–260. [DOI] [PubMed] [Google Scholar]
- Liu X. and Ferenci,T. (1998) Regulation of porin-mediated outer membrane permeability by nutrient limitation in Escherichia coli. J. Bacteriol., 180, 3917–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher K.P., Rees,B., Koebuik,R., Mitschler,A., Moulinier,L., Rosenbusch,J.P. and Moras,D. (1998) Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell, 95, 771–778. [DOI] [PubMed] [Google Scholar]
- Lopatin A., Makhina,E.N. and Nichols,C.G. (1994) Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature, 372, 366–369. [DOI] [PubMed] [Google Scholar]
- Mannella C.A. (1997) Minireview: on the structure and gating mechanism of the mitochondrial channel, VDAC. J. Bioenerg. Biomembr., 29, 525–531. [DOI] [PubMed] [Google Scholar]
- Margulis L. (1970) Origin of Eukaryotic Cells. Yale University Press, New Haven, CT.
- McIntosh D.B., Woolley,D.G., Vilsen,B. and Andersen,J.P. (1996) Mutagenesis of segment 487Phe-Ser-Arg-Asp-Arg-Lys492 of sarco plasmic reticulum Ca2+-ATPase produces pumps defective in ATP binding. J. Biol. Chem., 271, 25778–25789. [DOI] [PubMed] [Google Scholar]
- Newton S.M.C. et al. (1997) Double mutagenesis of a positive charge cluster in the ligand-binding site of the ferric enterobactin receptor, FepA. Proc. Natl Acad. Sci. USA, 94, 4560–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. (1993) Transport across the bacterial outer membrane. J. Bioenerg. Biomembr., 25, 581–589. [DOI] [PubMed] [Google Scholar]
- Nikaido H. and Rosenberg,E.Y. (1981) Effect on solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J. Gen. Physiol., 77, 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. and Saier,M.H. (1992) Transport proteins in bacteria: common themes in their design. Science, 258, 936–942. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nikaido,K. and Harayama,S. (1991) Identification and characterization of porins in Pseudomonas aeruginosa. J. Biol. Chem., 266, 770–779. [PubMed] [Google Scholar]
- Pohlmeyer K., Soll,J., Steinkamp,T., Hinnah,S. and Wagner,R. (1997) Isolation and characterization of an amino acid-selective channel protein present in the chloroplastic outer envelope membrane. Proc. Natl Acad. Sci. USA, 94, 9504–9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmeyer K., Soll,J., Grimm,R., Hill,K. and Wagner,R. (1998) A high-conductance solute channel in the chloroplastic outer envelope from pea. Plant Cell, 10, 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin I.I. (1992) Single channel recording in the chloroplast envelope. FEBS Lett., 308, 87–90. [DOI] [PubMed] [Google Scholar]
- Rassow J., Dekker,P.J.T., van Wilpe,S., Meijer,M. and Soll,J. (1999) The preprotein translocase of the mitochondrial inner membrane: function and evolution. J. Mol. Biol., 286, 105–120. [DOI] [PubMed] [Google Scholar]
- Röhl T., Motzkus,M. and Soll,J. (1999) The outer envelope protein OEP24 from pea chloroplasts can functionally replace the mitochondrial VDAC in yeast. FEBS Lett., 460, 491–494. [DOI] [PubMed] [Google Scholar]
- Rostovtseva T. and Colombini,M. (1996) ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane. J. Biol. Chem., 271, 28006–28008. [DOI] [PubMed] [Google Scholar]
- Rudel T., Schmid,A., Benz,R., Kolb,H.A., Lang,F. and Meyer,T.F. (1996) Modulation of Neisseria porin (PorB) by cytosolic ATP/GTP of target cells: parallels between pathogen accommodation and mitochondrial endosymbiosis. Cell, 85, 391–402. [DOI] [PubMed] [Google Scholar]
- Samartzidou H. and Delcour,A.H. (1998) E.coli PhoE porin has an opposite voltage-dependence to the homologous OmpF. EMBO J., 17, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samartzidou H. and Delcour,A.H. (1999) Excretion of endogenous cadaverine leads to a decrease in porin-mediated outer membrane permeability. J Bacteriol., 181, 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H. and Rosenbusch,J.P. (1978) Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc. Natl Acad. Sci. USA, 75, 3751–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R.H., Sutton,K.G. and Dolphin,A.C. (1993) Interactions of polyamines with neuronal ion channels. Trends Neurosci., 16, 153–160. [DOI] [PubMed] [Google Scholar]
- Sen K., Hellmann,J. and Nikaido,H. (1988) Porin channels in intact cells of Escherichia coli are not effected by Donnan potentials across the outer membrane. J. Biol. Chem., 263, 1182–1187. [PubMed] [Google Scholar]
- Shimizu S., Konishi,A., Kodama,T. and Tsujimoto,Y. (2000) BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc. Natl Acad. Sci. USA, 97, 3100–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare J.T., Ahmer,B.M.M., Seachord,C.L. Darveau,R.P. and Postle,K. (1993) Energy transduction between membranes: TonB, a cytoplasmic membrane protein, can be chemically crosslinked in vivo to the outer membrane receptor FepA. J. Biol. Chem., 268, 16302–16308. [PubMed] [Google Scholar]
- Soll J., Bölter,B., Wagner,R. and Hinnah,S.C. (2000) Transport in and out of plastids: does the outer envelope membrane control the flow? Trends Plant Sci., 5, 135–138. [DOI] [PubMed] [Google Scholar]
- Steinkamp T., Hill,K., Hinnah,S.C., Wagner,R., Röhl,T., Pohlmeyer,K. and Soll,J. (2000) Identification of the pore-forming region of the outer chloroplast envelope protein OEP16. J. Biol. Chem., 275, 11758–11764. [DOI] [PubMed] [Google Scholar]
- Sugawara E. and Nikaido,H. (1992) Pore-forming activity of OmpA protein of Escherichia coli. J. Biol. Chem., 267, 2507–2511. [PubMed] [Google Scholar]
- Thomas L., Kocsis,E., Colombini,M., Erbe,E., Trus,B.L. and Steven,A.C. (1991) Surface topography and molecular stoichiometry of the mitochondrial channel, VDAC, in crystalline arrays. J. Struct. Biol., 106, 161–171. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Overduin,P., Lugtenberg,B. and Bergmans,H. (1982) Cloning of phoE, the structural gene for the Escherichia coli phosphate limitation-inducible outer membrane pore protein. J. Bacteriol., 149, 668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara A., Fill,M., Velez,P., Yasukochi,M. and Imanaga,I. (1996) Rectification of rabbit cardiac ryanodine receptor current by endogenous polyamines. Biophys. J., 71, 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M.G., Chandel,S.N., Li,X.X., Schumacker,P.T., Colombini,M. and Thompson,C.B. (2000) Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc. Natl Acad. Sci. USA, 97, 4666–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.C. and Newton,A. (1969) Iron transport in Escherichia coli: roles of energy-dependent uptake and 2,3-dihydroxybenzoylserine. J. Bacteriol., 98, 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]