Abstract
Immunodeficiency syndromes associated with protein-energy malnutrition (PEM) have been documented extensively, although to date the mechanism underlying these defects remains uncharacterized. In this study, we have evaluated T, B, and antigen-presenting cell functions of malnourished mice fed a 4% protein diet compared with litter-mate controls fed a 20% protein diet. Spleen cells from malnourished mice presented both soluble foreign protein and allogeneic MHC antigens less efficiently than control mice. However, T cells from malnourished animals demonstrated effective or enhanced specific T-cell activation when stimulated with allogeneic cells, while B cells from protein-deprived animals responded normally in proliferative responses to T-cell driven cognate and non-cognate, as well as mitogen, stimulation. To assess further antigen-presenting cell function, three requirements for successful antigen presentation were evaluated. First, the proliferation of the IL-1-dependent cloned T-cell line D10 demonstrated a slight deficiency in IL-1 production by malnourished splenic antigen-presenting cells, and the addition of saturating amounts of IL-1 to the assay could partially reconstitute function. Second, quantitative cell-sorter analysis revealed minimal deficiencies of spleen-cell Ia expression. Third, antigen-processing function was assayed in vitro by using processed antigen fragments; no improvement in protein-deprived antigen-presenting function resulted. Together, these findings suggest that either decreased Ia glycoprotein expression on a critical subset of antigen-presenting cells (APCs) or a quantitative deficiency in such a subset of cells, or both, underlie the defective antigen-presenting cell function observed in chronic protein deprivation (CPD).
Full text
PDF

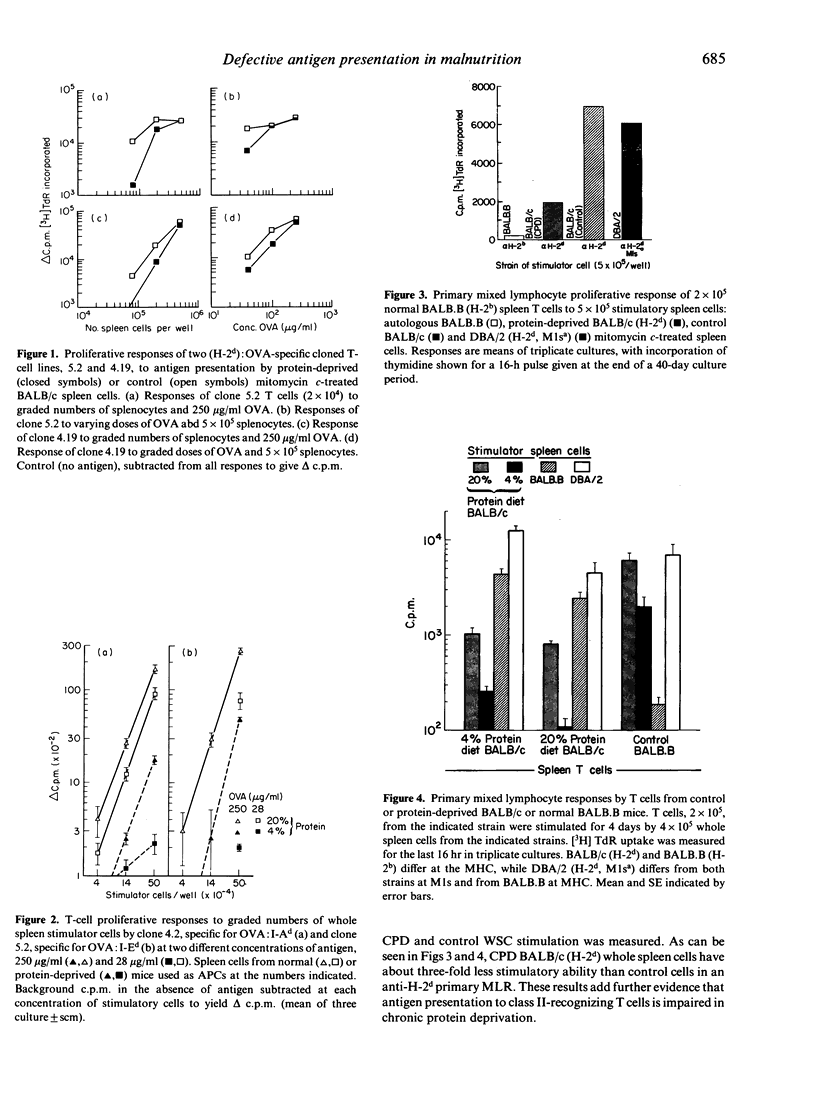
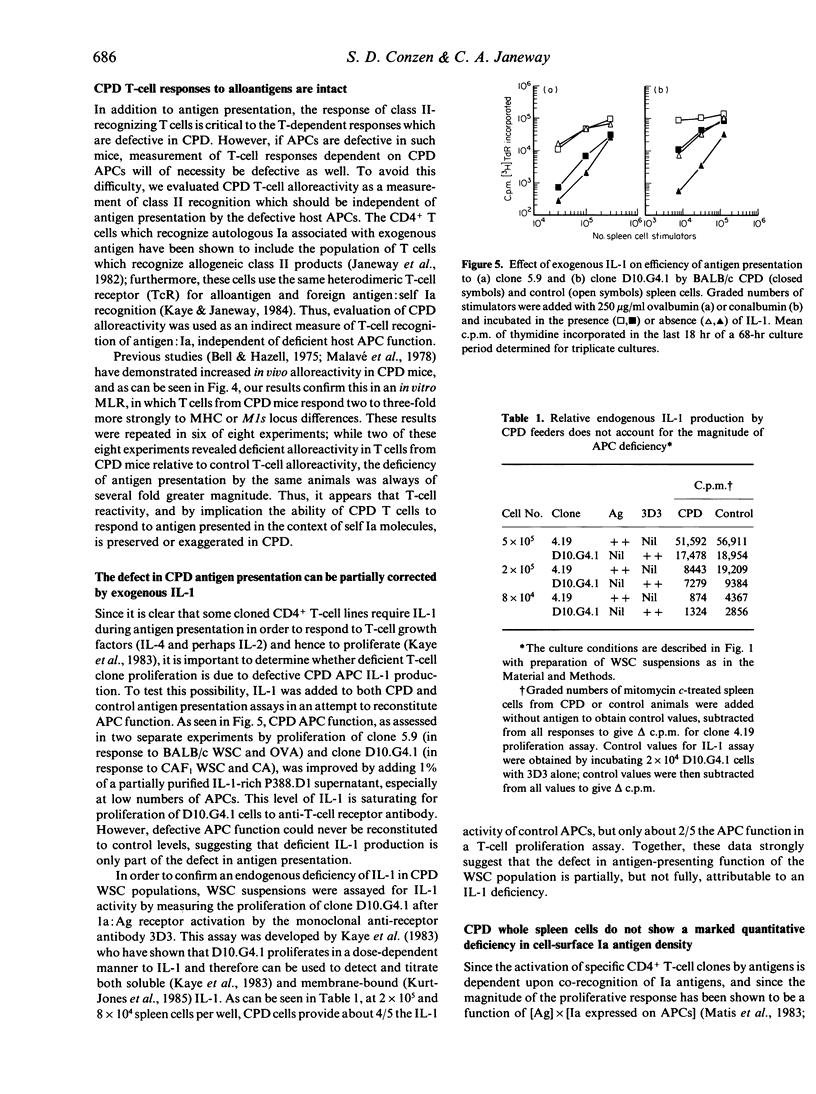
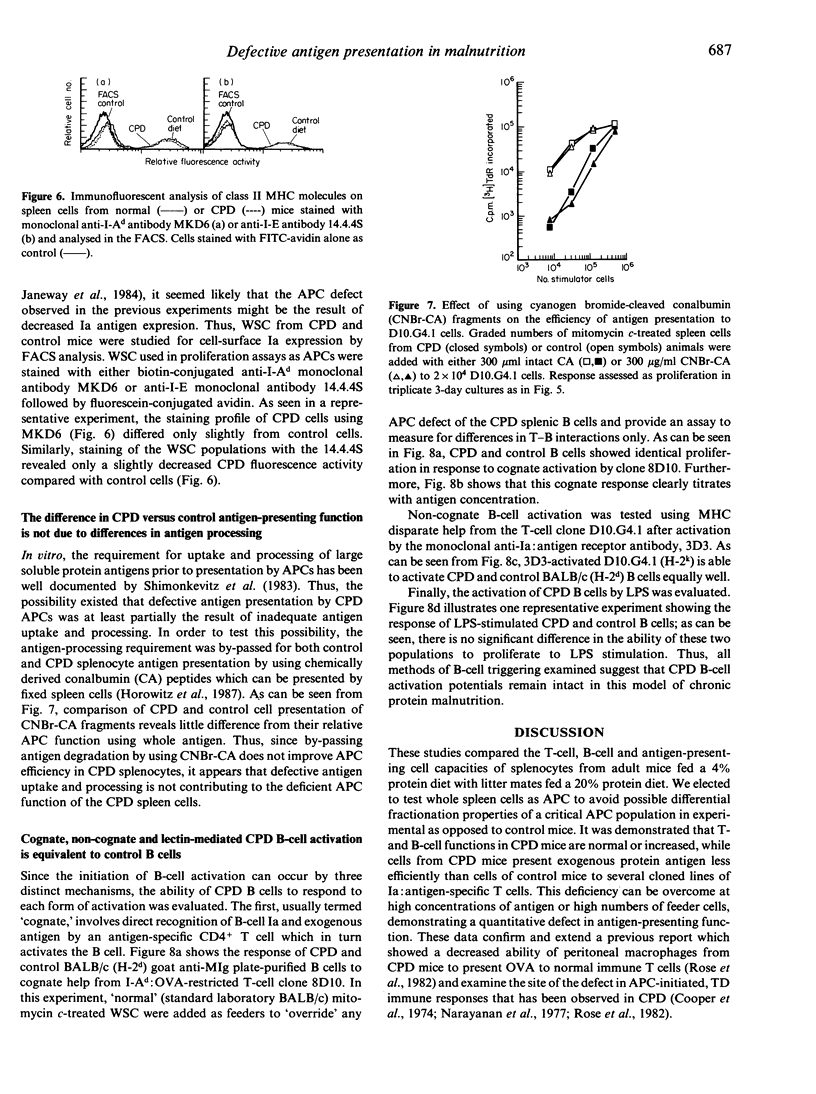

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Wilchek M., Skutelsky E. Affinity cytochemistry: the localization of lectin and antibody receptors on erythrocytes via the avidin-biotin complex. FEBS Lett. 1976 Oct 1;68(2):240–244. doi: 10.1016/0014-5793(76)80445-0. [DOI] [PubMed] [Google Scholar]
- Bell R. G., Hazell L. A. Influence of dietary protein restriction on immune competence. I. Effect on the capacity of cells from various lymphoid organs to induce graft-vs.-host reactions. J Exp Med. 1975 Jan 1;141(1):127–137. doi: 10.1084/jem.141.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. G., Hazell L. A., Price P. Influence of dietary protein restriction on immune competence. II. Effect on lymphoid tissue. Clin Exp Immunol. 1976 Nov;26(2):314–326. [PMC free article] [PubMed] [Google Scholar]
- Bhaskaram P., Sivakumar B. Interleukin-1 in malnutrition. Arch Dis Child. 1986 Feb;61(2):182–185. doi: 10.1136/adc.61.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper W. C., Good R. A., Mariani T. Effects of protein insufficiency on immune responsiveness. Am J Clin Nutr. 1974 Jun;27(6):647–664. doi: 10.1093/ajcn/27.6.647. [DOI] [PubMed] [Google Scholar]
- Coward W. A., Whitehead R. G., Lunn P. G. Reasons why hypoalbuminaemia may or may not appear in protein-energy malnutrition. Br J Nutr. 1977 Jul;38(1):115–126. doi: 10.1079/bjn19770067. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Gershon R. K. Interleukin 1 can replace the requirement for I-A-positive cells in the proliferation of antigen-primed T cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4747–4750. doi: 10.1073/pnas.79.15.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. B., Kaye J., Katz M. E., Janeway C. A., Jr Ability of fixed B-lymphoma cells to present foreign protein antigen fragments and allogenic MHC molecules to a cloned helper-T-cell line. Cell Immunol. 1987 Oct 15;109(2):429–436. doi: 10.1016/0008-8749(87)90325-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J., Janeway C. A., Jr The Fab fragment of a directly activating monoclonal antibody that precipitates a disulfide-linked heterodimer from a helper T cell clone blocks activation by either allogeneic Ia or antigen and self-Ia. J Exp Med. 1984 May 1;159(5):1397–1412. doi: 10.1084/jem.159.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J., Rollwagen F., Asofsky R., Lefkovits I. The abnormal function of T cells in chronically anti-mu-treated mice with no mature B lymphocytes. Eur J Immunol. 1984 May;14(5):476–482. doi: 10.1002/eji.1830140517. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Malavé I., Németh A., Blanca I. Immune response in malnutrition. Effect of protein deficiency on the DNA synthetic response to alloantigens. Int Arch Allergy Appl Immunol. 1978;56(2):128–135. doi: 10.1159/000232015. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Glimcher L. H., Paul W. E., Schwartz R. H. Magnitude of response of histocompatibility-restricted T-cell clones is a function of the product of the concentrations of antigen and Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6019–6023. doi: 10.1073/pnas.80.19.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis L. A., Jones P. P., Murphy D. B., Hedrick S. M., Lerner E. A., Janeway C. A., Jr, McNicholas J. M., Schwartz R. H. Immune response gene function correlates with the expression of an Ia antigen. II. A quantitative deficiency in Ae:E alpha complex expression causes a corresponding defect in antigen-presenting cell function. J Exp Med. 1982 Feb 1;155(2):508–523. doi: 10.1084/jem.155.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R. B., Nath I., Bhuyan U. N., Talwar G. P. Depression of T-cell function and normality of B-cell response in protein calorie malnutrition. Immunology. 1977 Mar;32(3):345–350. [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Rose A. H., Holt P. G., Turner K. J. The effect of a low protein diet on the immunogenic activity of murine peritoneal macrophages. Int Arch Allergy Appl Immunol. 1982;67(4):356–361. doi: 10.1159/000233047. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Whitehead R. G., Alleyne G. A. Pathophysiological factors of importance in protein-calorie malnutrition. Br Med Bull. 1972 Jan;28(1):72–79. doi: 10.1093/oxfordjournals.bmb.a070897. [DOI] [PubMed] [Google Scholar]
- Williams J., Elleman T. C., Kingston I. B., Wilkins A. G., Kuhn K. A. The primary structure of hen ovotransferrin. Eur J Biochem. 1982 Feb;122(2):297–303. doi: 10.1111/j.1432-1033.1982.tb05880.x. [DOI] [PubMed] [Google Scholar]