Abstract
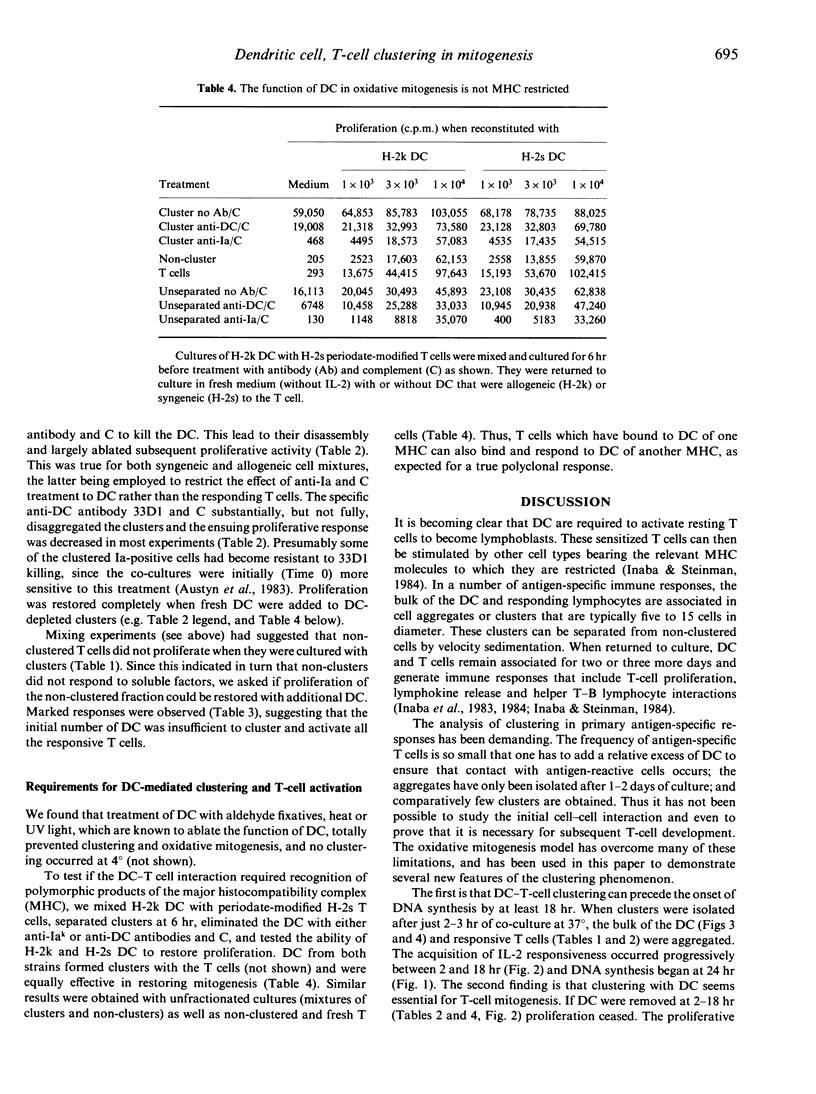
Several antigen-specific immune responses are known to occur in discrete aggregates of dendritic cells (DC) and lymphocytes. We have used a polyclonal model, the mitogenesis of T cells that have been modified with sodium periodate, to evaluate the significance of cell-cell clustering. Firstly, we found that clustering precedes the onset of DNA synthesis by a day. Within 2 hr, virtually all of the added dendritic cells and most of the T cells that will respond have formed clusters. The T cells then progressively release and become responsive to interleukin-2 over 18 hr and DNA synthesis begins at 24 hr. Secondly, clustering with dendritic cells appears to be essential for mitogenesis. If dendritic cells are eliminated, the clusters disassemble and subsequent proliferation is reduced. Clustering and proliferation can be restored with dendritic cells that are syngeneic or allogeneic with the initial inoculum. DC are inactive if they are treated with ultraviolet light, formaldehyde or heat. Thirdly, the non-clustered cells do not synthesize DNA even when mixed with the clusters. However, non-clusters will respond when supplemented with additional DC. We conclude that clustering with DC precedes and seems essential for T-cell mitogenesis in the periodate model.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Morris P. J. T-cell activation by dendritic cells: CD18-dependent clustering is not sufficient for mitogenesis. Immunology. 1988 Mar;63(3):537–543. [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M., Steinman R. M., Weinstein D. E., Granelli-Piperno A., Palladino M. A. Dendritic cells initiate a two-stage mechanism for T lymphocyte proliferation. J Exp Med. 1983 Apr 1;157(4):1101–1115. doi: 10.1084/jem.157.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greineder D. K., Rosenthal A. S. The requirement for macrophage-lymphocyte interaction in T lymphocyte proliferation induced by generation of aldehydes on cell membranes. J Immunol. 1975 Oct;115(4):932–938. [PubMed] [Google Scholar]
- Greineder D. K., Shevach E. M., Rosenthal A. S. Macrophage-lymphocyte interaction. III. Site of alloantiserum inhibition of T lymphocyte proliferation induced by allogeneic or aldehyde-bearing cells. J Immunol. 1976 Oct;117(4):1261–1266. [PubMed] [Google Scholar]
- Inaba K., Granelli-Piperno A., Steinman R. M. Dendritic cells induce T lymphocytes to release B cell-stimulating factors by an interleukin 2-dependent mechanism. J Exp Med. 1983 Dec 1;158(6):2040–2057. doi: 10.1084/jem.158.6.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Witmer M. D., Steinman R. M. Clustering of dendritic cells, helper T lymphocytes, and histocompatible B cells during primary antibody responses in vitro. J Exp Med. 1984 Sep 1;160(3):858–876. doi: 10.1084/jem.160.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., O'Brien J. P., Beyer C. F., Bowers W. E. Rat dendritic cells function as accessory cells and control the production of a soluble factor required for mitogenic responses of T lymphocytes. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5414–5418. doi: 10.1073/pnas.77.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Witmer M. D., Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):161–165. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Hill S. W., Parker J. W., O'Brien R. L., Frelinger J. A. Characterization of responding cells in oxidative mitogen stimulation. III. Presence of I-A- and I-J, E, C-subregion gene products on the surface of required cells. Immunogenetics. 1980;10(2):133–140. doi: 10.1007/BF01561562. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Parker J. W., Frelinger J. A., O'Brien R. L. Characterization of responding cells in oxidative mitogen stimulation. II. Identification of an Ia-bearing adherent accessory cell. J Immunol. 1980 Jun;124(6):2700–2707. [PubMed] [Google Scholar]
- Pugh C. W., MacPherson G. G., Steer H. W. Characterization of nonlymphoid cells derived from rat peripheral lymph. J Exp Med. 1983 Jun 1;157(6):1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Nogueira N., Witmer M. D., Tydings J. D., Mellman I. S. Lymphokine enhances the expression and synthesis of Ia antigens on cultured mouse peritoneal macrophages. J Exp Med. 1980 Nov 1;152(5):1248–1261. doi: 10.1084/jem.152.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Valinsky J., Hoffman E., Luban J., Hair L. S., Steinman R. M. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983 Jul 1;158(1):174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]