Abstract
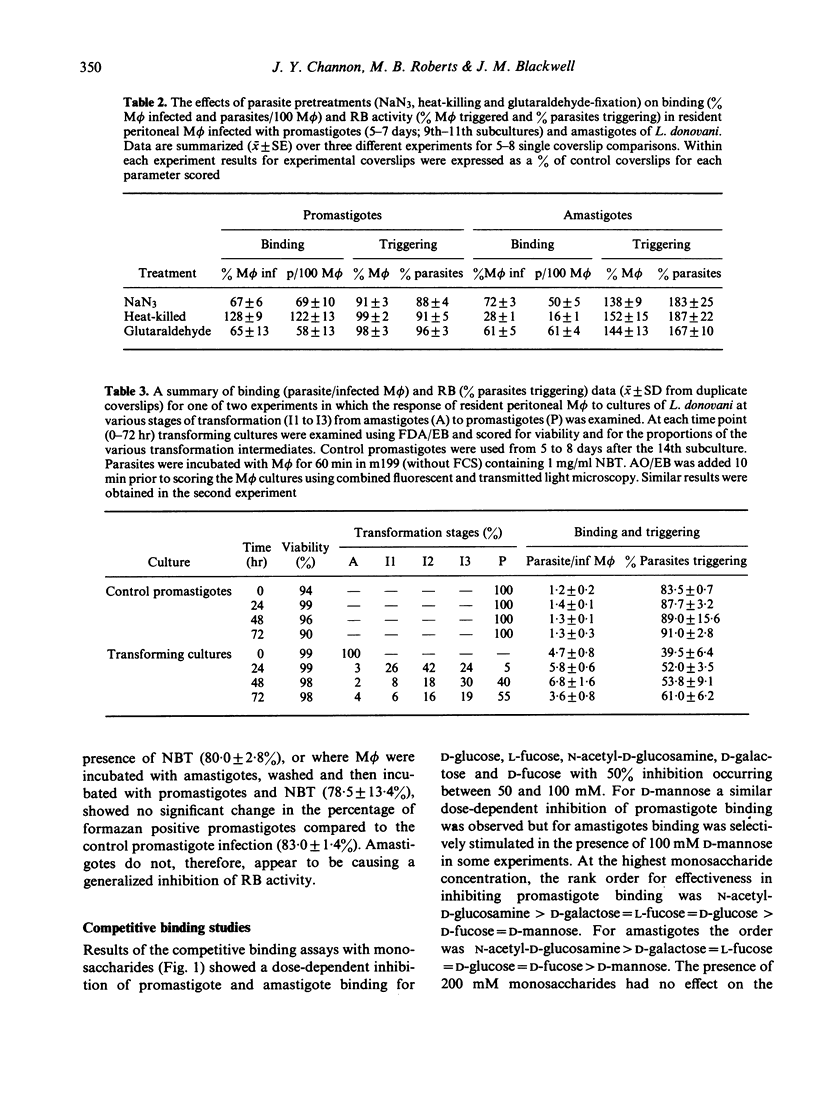
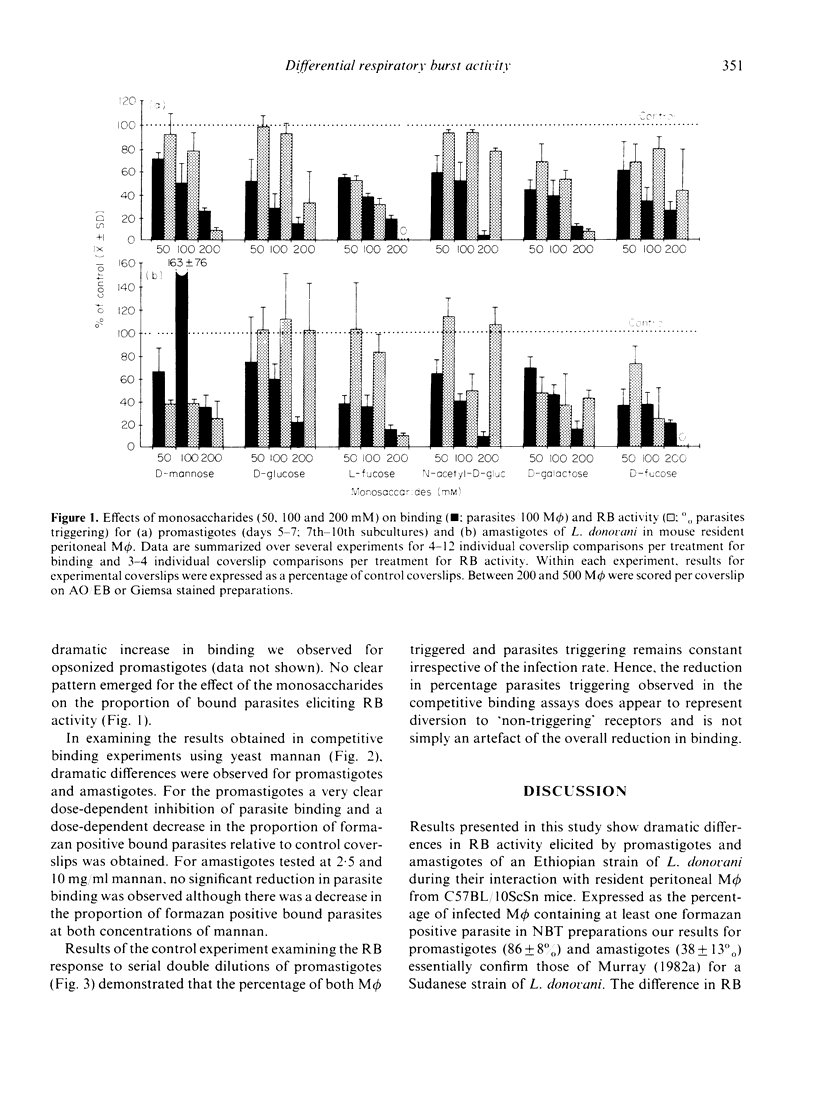
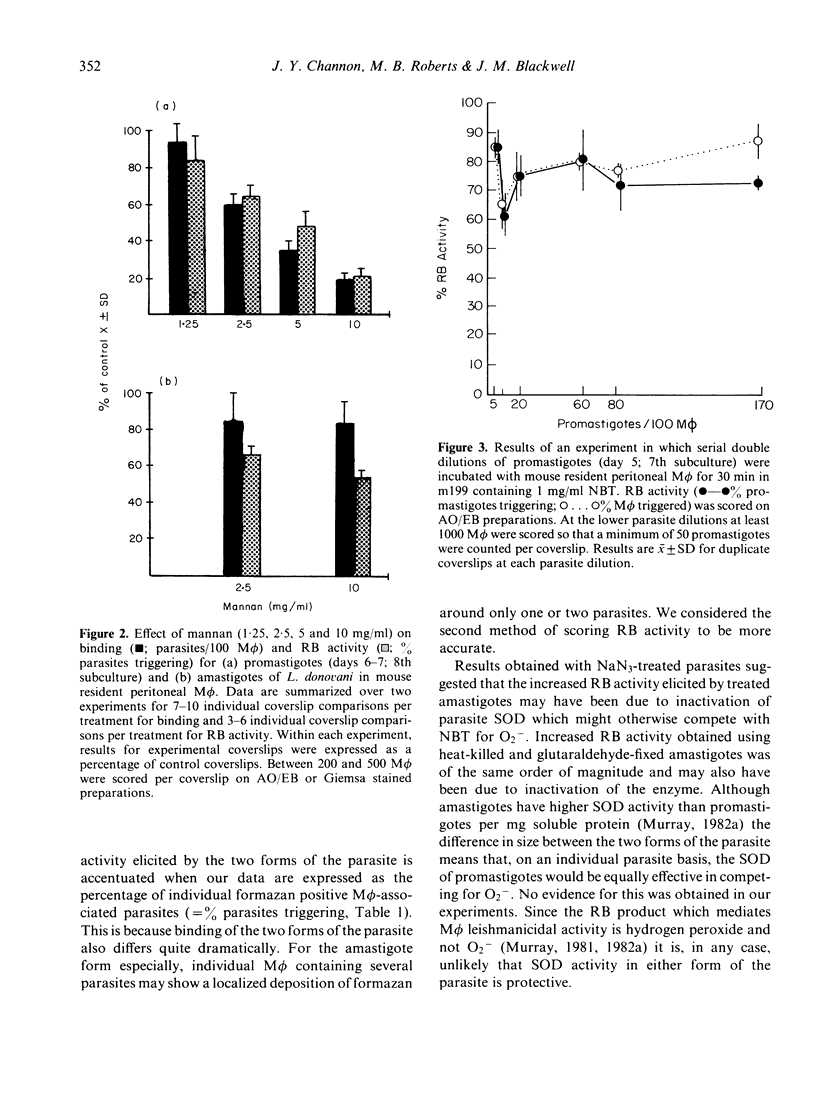
Acridine orange and ethidium bromide and a combination of fluorescent and transmitted light microscopy used in conjunction with the qualitative nitroblue tetrazolium assay for superoxide anion (O2-) release demonstrated dramatic differences in the binding of and respiratory burst (RB) activity elicited by promastigotes and amastigotes of Leishmania donovani in resident peritoneal macrophages (M phi) from C57BL/10ScSn mice. When amastigotes were incubated with M phi for 30 min the number of parasites per 100 M phi was 2-4-fold higher, a higher proportion of M phi became infected and the mean number of parasites per infected M phi was higher than in promastigote infections. RB activity was higher for promastigotes than amastigotes both in terms of the percentage of infected M phi containing formazan positive parasites and the percentage of individual formazan positive parasites. In an attempt to explain the differential response to promastigotes and amastigotes, RB activity was examined for sodium azide-treated, glutaraldehyde-fixed and heat-killed parasites and for various transformation intermediates between amastigotes and promastigotes. Binding and RB activity were also examined in conjunction with competitive binding assays designed to determine the specific receptors involved in ligand binding of both forms of the parasite to the M phi. The results indicate that, while amastigotes may possess an azide-sensitive mechanism which either competes for O2- produced or causes localized inactivation of RB activity, this cannot account for the full magnitude of the difference between the two forms of the parasite. The transformation and competitive binding studies suggest that the more likely explanation lies in both qualitative and quantitative differences in the distribution of surface ligands involved in binding the parasite to the M phi plasma membrane and that the well characterized mannose/fucose receptor may be important in promastigote, but not amastigote, binding and RB activity.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Vickerman K. Fusion of host cell secondary lysosomes with the parasitophorous vacuoles of Leishmania mexicana-infected macrophages. J Protozool. 1975 Nov;22(4):502–508. doi: 10.1111/j.1550-7408.1975.tb05219.x. [DOI] [PubMed] [Google Scholar]
- Berton G., Gordon S. Modulation of macrophage mannosyl-specific receptors by cultivation on immobilized zymosan. Effects on superoxide-anion release and phagocytosis. Immunology. 1983 Aug;49(4):705–715. [PMC free article] [PubMed] [Google Scholar]
- Brun R., Berens R. L., Krassner S. M. Inhibition of Leishmania donovani transformation by hamster spleen homogenates and active human lymphocytes. Nature. 1976 Aug 19;262(5570):689–691. doi: 10.1038/262689a0. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Leishmania donovani-macrophage binding mediated by surface glycoproteins/antigens: characterization in vitro by a radioisotopic assay. Mol Biochem Parasitol. 1981 Nov;4(1-2):67–76. doi: 10.1016/0166-6851(81)90030-x. [DOI] [PubMed] [Google Scholar]
- Crane M. S., Dvorak J. A. Influence of monosaccharides on the infection of vertebrate cells by Trypanosoma cruzi and Toxoplasma gondii. Mol Biochem Parasitol. 1982 May;5(5):333–341. doi: 10.1016/0166-6851(82)90040-8. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. Leishmania donovani: surface membrane carbohydrates of promastigotes. Exp Parasitol. 1977 Apr;41(2):341–358. doi: 10.1016/0014-4894(77)90107-2. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., Hill M., Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984 Jan 1;159(1):244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass E., Stewart J., Weir D. M. Presence of bacterial binding 'lectin-like' receptors on phagocytes. Immunology. 1981 Nov;44(3):529–534. [PMC free article] [PubMed] [Google Scholar]
- Hoshino-Shimizu S., Mineo J. R., Camargo M. E. Lectin used in the purification process of Toxoplasma gondii tachyzoites. J Parasitol. 1980 Dec;66(6):989–991. [PubMed] [Google Scholar]
- Jones T. C., Yeh S., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. I. Mechanism of entry and intracellular fate of the parasite. J Exp Med. 1972 Nov 1;136(5):1157–1172. doi: 10.1084/jem.136.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzin A. M., Colli W. Lectin receptors in Trypanosoma cruzi. An N-acetyl-D-glucosamine-containing surface glycoprotein specific for the trypomastigote stage. Biochim Biophys Acta. 1983 Jan 19;727(2):403–411. doi: 10.1016/0005-2736(83)90425-x. [DOI] [PubMed] [Google Scholar]
- Meshnick S. R., Eaton J. W. Leishmanial superoxide dismutase: a possible target for chemotherapy. Biochem Biophys Res Commun. 1981 Oct 15;102(3):970–976. doi: 10.1016/0006-291x(81)91633-8. [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Roberts J. F. Trypanosoma brucei: recognition in vitro of two developmental forms by murine macrophages. Exp Parasitol. 1982 Dec;54(3):310–316. doi: 10.1016/0014-4894(82)90040-6. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol. 1982 Jul;129(1):351–357. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Pretreatment with phorbol myristate acetate inhibits macrophage activity against intracellular protozoa. J Reticuloendothel Soc. 1982 Jun;31(6):479–487. [PubMed] [Google Scholar]
- Murray H. W. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med. 1981 May 1;153(5):1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamura Y., Kolb H. Presence of a lectin-like receptor for D-galactose on rat peritoneal macrophages. FEBS Lett. 1980 Jun 16;115(1):59–62. doi: 10.1016/0014-5793(80)80726-5. [DOI] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogmundsdóttir H. M., Weir D. M. The characteristics of binding of Corynebacterium parvum to glass-adherent mouse peritoneal exudate cells. Clin Exp Immunol. 1976 Nov;26(2):334–339. [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Rahman A., Pelster B., Brandis H. Search for the presence of lectin-binding sites on Toxoplasma gondii. J Parasitol. 1977 Dec;63(6):1076–1080. [PubMed] [Google Scholar]
- Shepherd V. L., Lee Y. C., Schlesinger P. H., Stahl P. D. L-Fucose-terminated glycoconjugates are recognized by pinocytosis receptors on macrophages. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1019–1022. doi: 10.1073/pnas.78.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger R. F., Black C. D. Simplified defined media for cultivating Leishmania donovani promastigotes. Acta Trop. 1980 Jun;37(2):195–198. [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983 Jan;96(1):160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teradaira R., Kolb-Bachofen V., Schlepper-Schäfer J., Kolb H. Galactose-particle receptor on liver macrophages. Quantitation of particle uptake. Biochim Biophys Acta. 1983 Sep 13;759(3):306–310. doi: 10.1016/0304-4165(83)90329-x. [DOI] [PubMed] [Google Scholar]
- Townsend R., Stahl P. Isolation and characterization of a mannose/N-acetylglucosamine/fucose-binding protein from rat liver. Biochem J. 1981 Jan 15;194(1):209–214. doi: 10.1042/bj1940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehavi U., El-On J., Pearlman E., Abrahams J. C., Greenblatt C. L. Binding of Leishmania promastigotes to macrophages. Z Parasitenkd. 1983;69(4):405–414. doi: 10.1007/BF00927696. [DOI] [PubMed] [Google Scholar]
- Zenian A., Kierszenbaum F. Inhibition of macrophage-Trypanosoma cruzi interaction by concanavalin A and differential binding of bloodstream and culture forms to the macrophage surface. J Parasitol. 1982 Jun;68(3):408–415. [PubMed] [Google Scholar]
- Zenian A., Kierszenbaum F. Trypanosoma cruzi: differences in cell surface interaction of circulating (trypomastigote) and culture (epimastigote) forms with macrophages. J Parasitol. 1983 Aug;69(4):660–665. [PubMed] [Google Scholar]


