Abstract
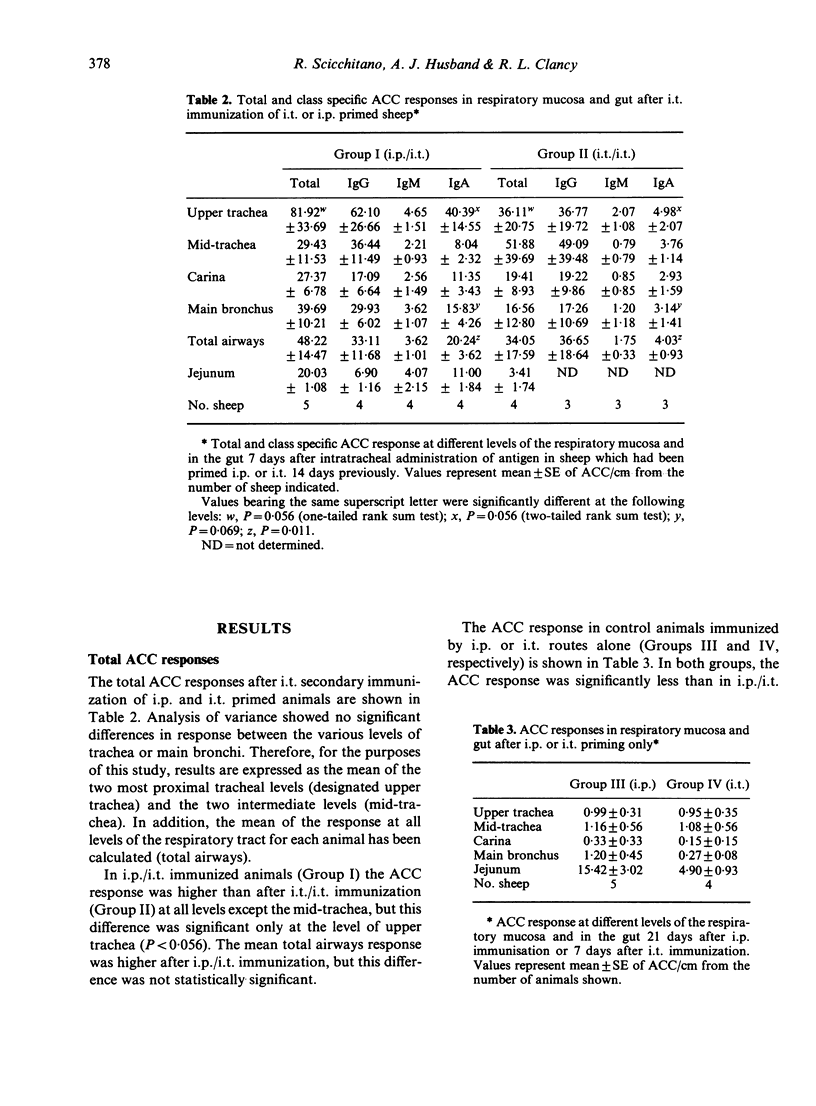
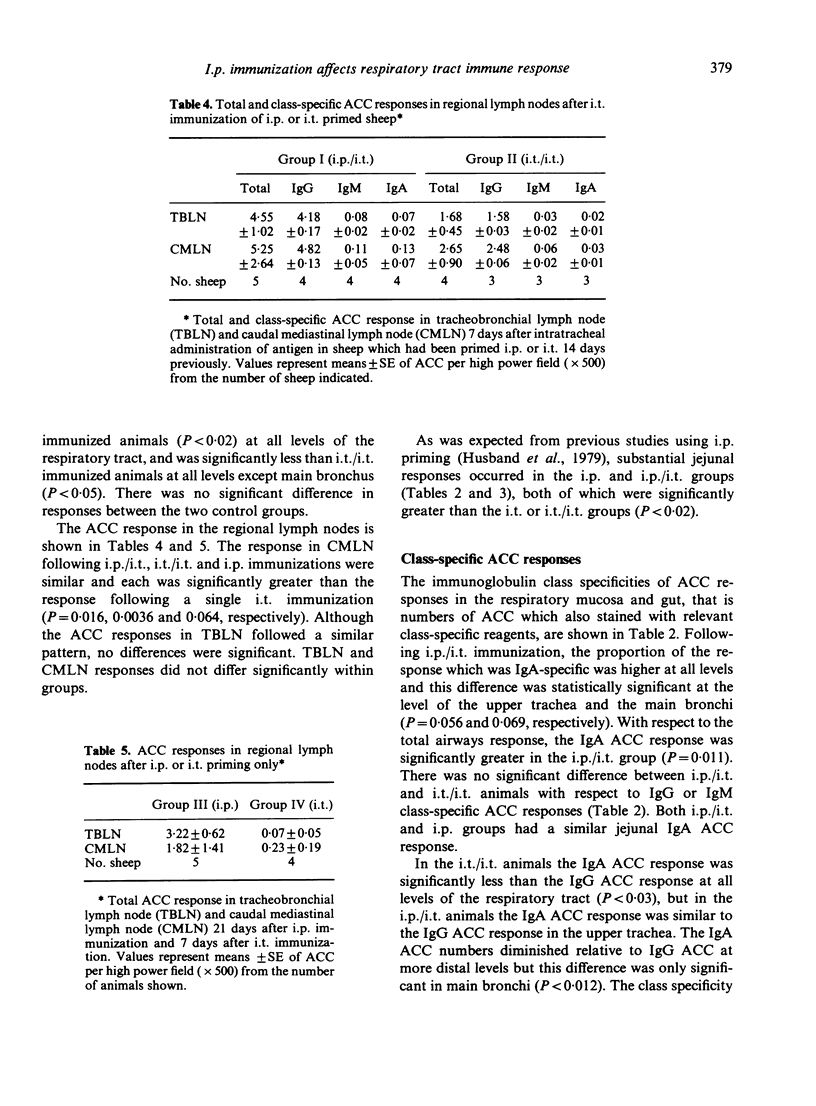
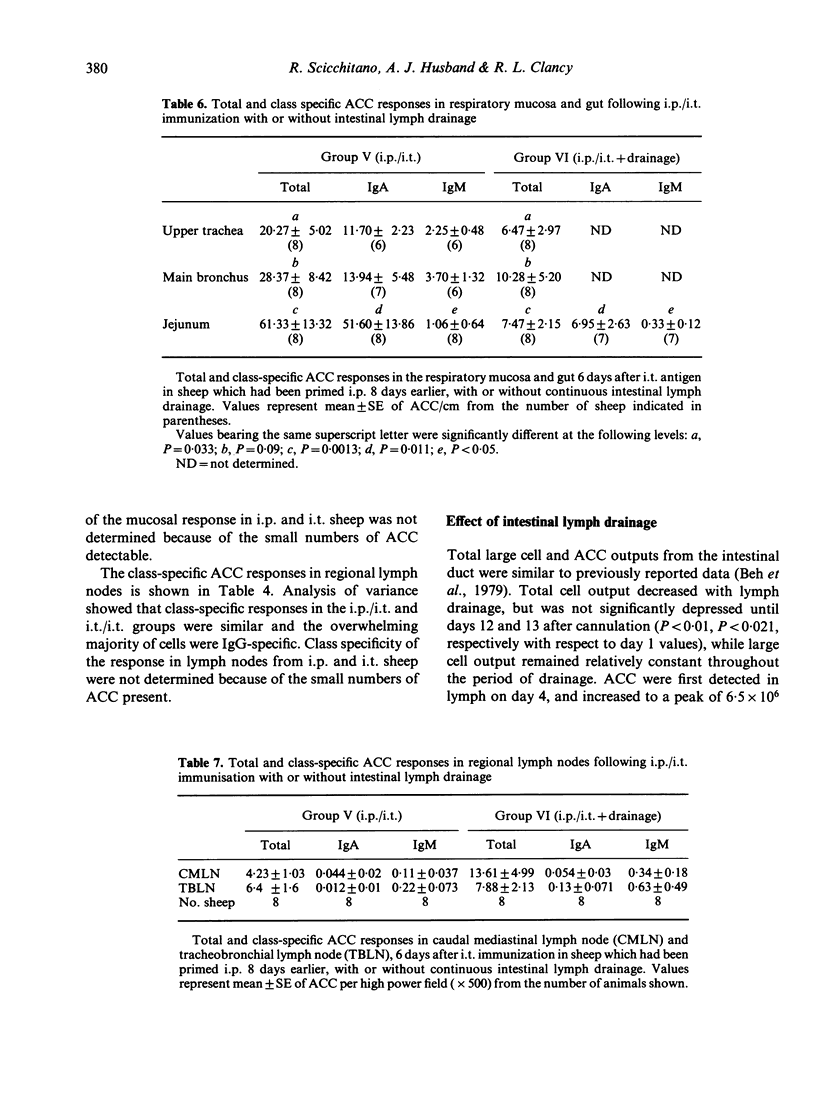
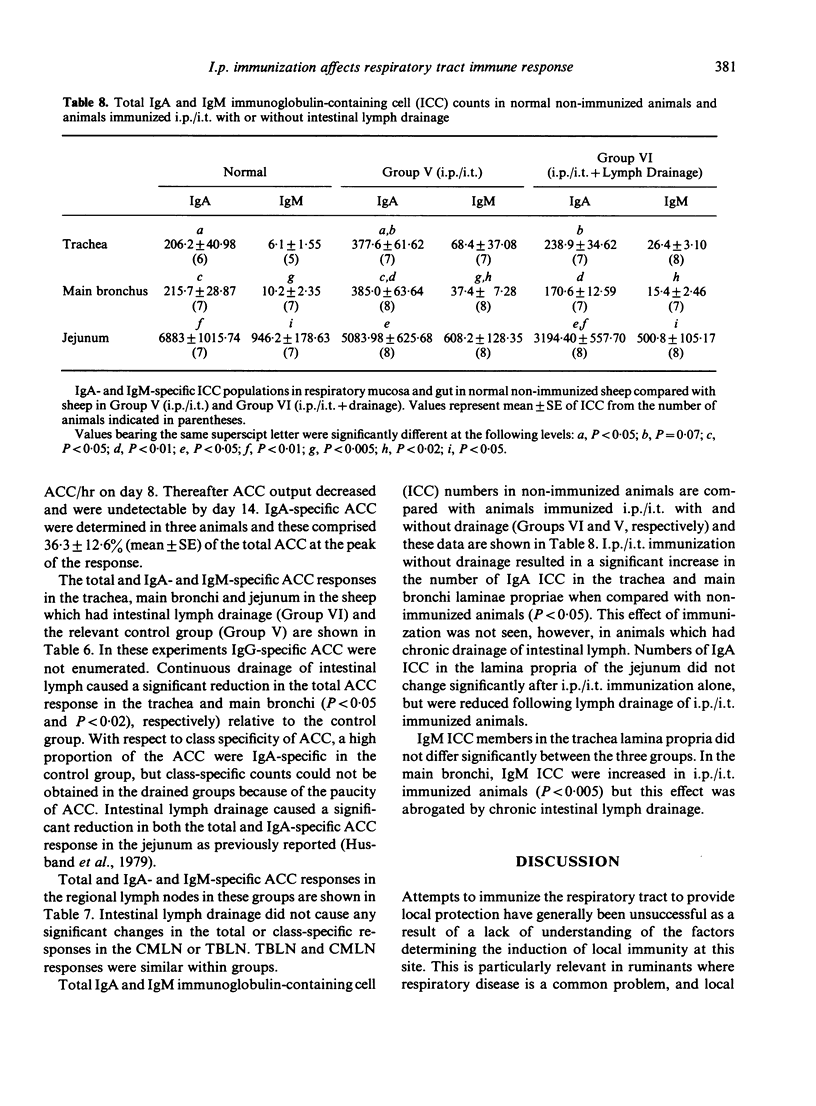
The contribution of gut-associated lymphoid tissue (GALT) to the local immune response in the respiratory mucosa of sheep has been investigated. Sheep were primed intraperitoneally (i.p.) with antigen in Freund's complete adjuvant, a procedure known to produce a large IgA-specific antibody-containing cell (ACC) response in intestinal lymph. ACC and their class specificity were then enumerated by double fluorochrome immunofluorescence in respiratory tissues after intratracheal (i.t.) antigen administration. This immunization procedure produced an enhanced IgA-specific ACC response in the upper respiratory tract mucosa compared with either i.t. or i.p. immunization alone and this was not reflected in the regional lymph nodes. Furthermore, chronic drainage of the intestinal efferent lymphatic duct for the duration of the immunization period abrogated the enhanced response in the respiratory mucosa. These data are consistent with the concept of an intermucosal cell circuit with respect to IgA cell precursors, and provide indirect evidence that IgA responses in the respiratory tract can be enhanced by harnessing the immune potential of GALT as a source of IgA precursors by appropriate immunization strategies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beh K. J., Husband A. J., Lascelles A. K. Intestinal response of sheep to intraperitoneal immunization. Immunology. 1979 Jun;37(2):385–388. [PMC free article] [PubMed] [Google Scholar]
- Beh K. J., Lascelles A. K. The effect of route of administration of antigen on the antibody-containing cell response in lymph of sheep. Immunology. 1981 Apr;42(4):577–582. [PMC free article] [PubMed] [Google Scholar]
- Beh K. J. The origin of IgA-containing cells in intestinal lymph of sheep. Aust J Exp Biol Med Sci. 1977 Jun;55(3):263–274. doi: 10.1038/icb.1977.21. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Harris D. L., Hill J. O., Muggenburg B. A., Wolff R. K. Immune responses after localized lung immunization in the dog. Am Rev Respir Dis. 1980 Nov;122(5):755–760. doi: 10.1164/arrd.1980.122.5.755. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973 Jun;28(6):686–692. [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps A. W., Husband A. J., Lascelles A. K. The origin of immunoglobulins in intestinal secretion of sheep. Aust J Exp Biol Med Sci. 1974 Aug;52(4):711–716. doi: 10.1038/icb.1974.70. [DOI] [PubMed] [Google Scholar]
- Gorin A. B., Stewart P., Gould J. Concentrations of immunoglobulin classes in subcompartments of the sheep lung. Res Vet Sci. 1979 Jan;26(1):126–128. [PubMed] [Google Scholar]
- Husband A. J. An immunisation model for the control of infectious enteritis. Res Vet Sci. 1978 Sep;25(2):173–177. [PubMed] [Google Scholar]
- Husband A. J., Beh K. J., Lascelles A. K. IgA-containing cells in the ruminant intestine following intraperitoneal and local immunization. Immunology. 1979 Jul;37(3):597–601. [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J. Kinetics of extravasation and redistribution of IgA-specific antibody-containing cells in the intestine. J Immunol. 1982 Mar;128(3):1355–1359. [PubMed] [Google Scholar]
- Johnston N. W., Bienenstock J. Abolition of non-specific fluorescent staining of eosinophils. J Immunol Methods. 1974 Mar;4(2):189–194. doi: 10.1016/0022-1759(74)90060-x. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B., Chan M. K. The class-specific immunoglobulin composition of fluids obtained from various levels of the canine respiratory tract. J Immunol. 1976 Feb;116(2):423–429. [PubMed] [Google Scholar]
- Kaltreider H. B., Kyselka L., Salmon S. E. Immunology of the lower respiratory tract. II. The plaque-forming response of canine lymphoid tissues to sheep erythrocytes after intrapulmonary or intravenous immunization. J Clin Invest. 1974 Aug;54(2):263–270. doi: 10.1172/JCI107761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES A. K., MORRIS B. Surgical techniques for the collection of lymph from unanaesthetized sheep. Q J Exp Physiol Cogn Med Sci. 1961 Jul;46:199–205. doi: 10.1113/expphysiol.1961.sp001536. [DOI] [PubMed] [Google Scholar]
- Lascelles A. K., McDowell G. H. Localized humoral immunity with particular reference to ruminants. Transplant Rev. 1974;19(0):170–208. doi: 10.1111/j.1600-065x.1974.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Lascelles A. K. Antibody-producing cells in antigenically stimulated mammary glands and in the gastro-intestinal tract of sheep. Aust J Exp Biol Med Sci. 1970 Oct;48(5):525–535. doi: 10.1038/icb.1970.52. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- Morgan K. L., Hussein A. M., Newby T. J., Bourne F. J. Quantification and origin of the immunoglobulins in porcine respiratory tract secretions. Immunology. 1980 Nov;41(3):729–736. [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzik O., Perey D. Y., Bienenstock J. Differential IgA repopulation after transfer of autologous and allogeneic rabbit Peyer's patch cells. J Immunol. 1975 Jan;114(1 Pt 1):40–44. [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- Scicchitano R., Husband A. J., Cripps A. W. Immunoglobulin-containing cells and the origin of immunoglobulins in the respiratory tract of sheep. Immunology. 1984 Jul;52(3):529–537. [PMC free article] [PubMed] [Google Scholar]
- Smith W. D., Dawson A. M., Wells P. W., Burrells C. Immunoglobulin concentrations in ovine body fluids. Res Vet Sci. 1975 Sep;19(2):189–194. [PubMed] [Google Scholar]
- Stein-Streilein J., Hart D. A. Effect of route of immunization on development of antibody-forming cells in hilar lymph nodes. Infect Immun. 1980 Nov;30(2):391–396. doi: 10.1128/iai.30.2.391-396.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1–96. doi: 10.1016/s0065-2776(08)60441-1. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]


