Abstract
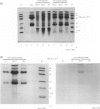
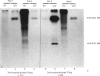
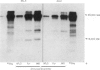
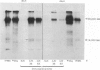
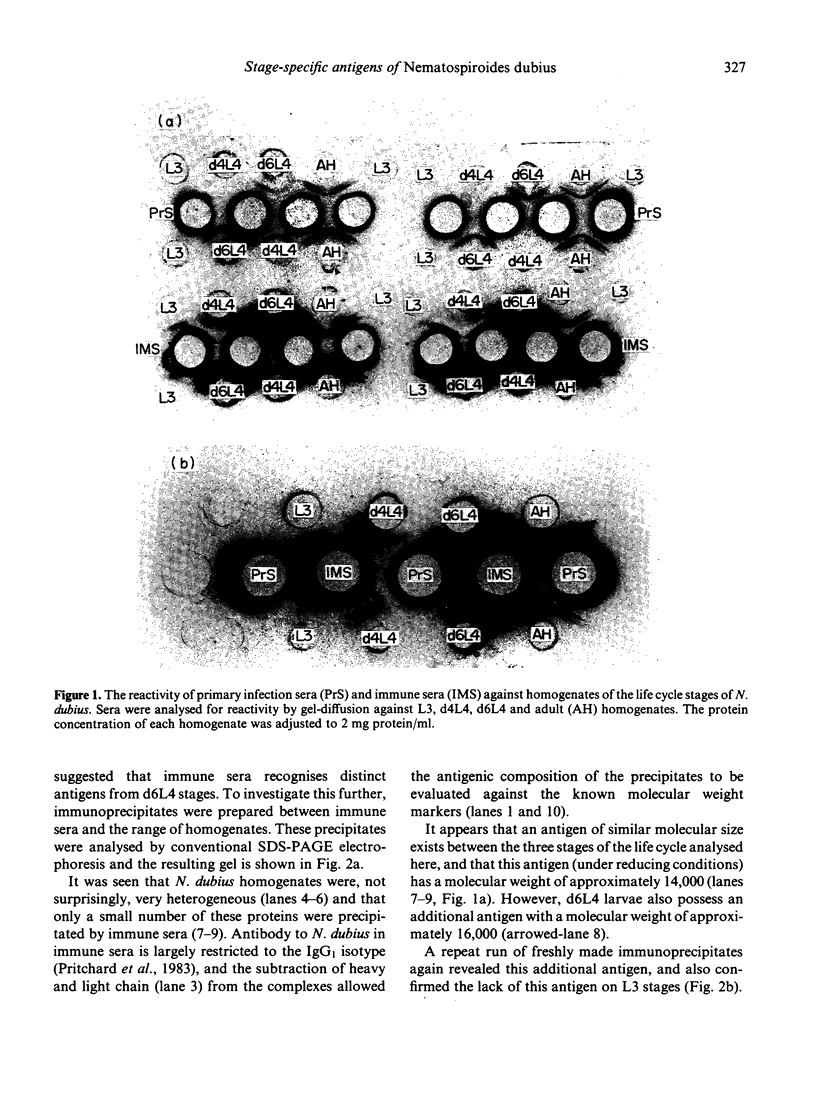
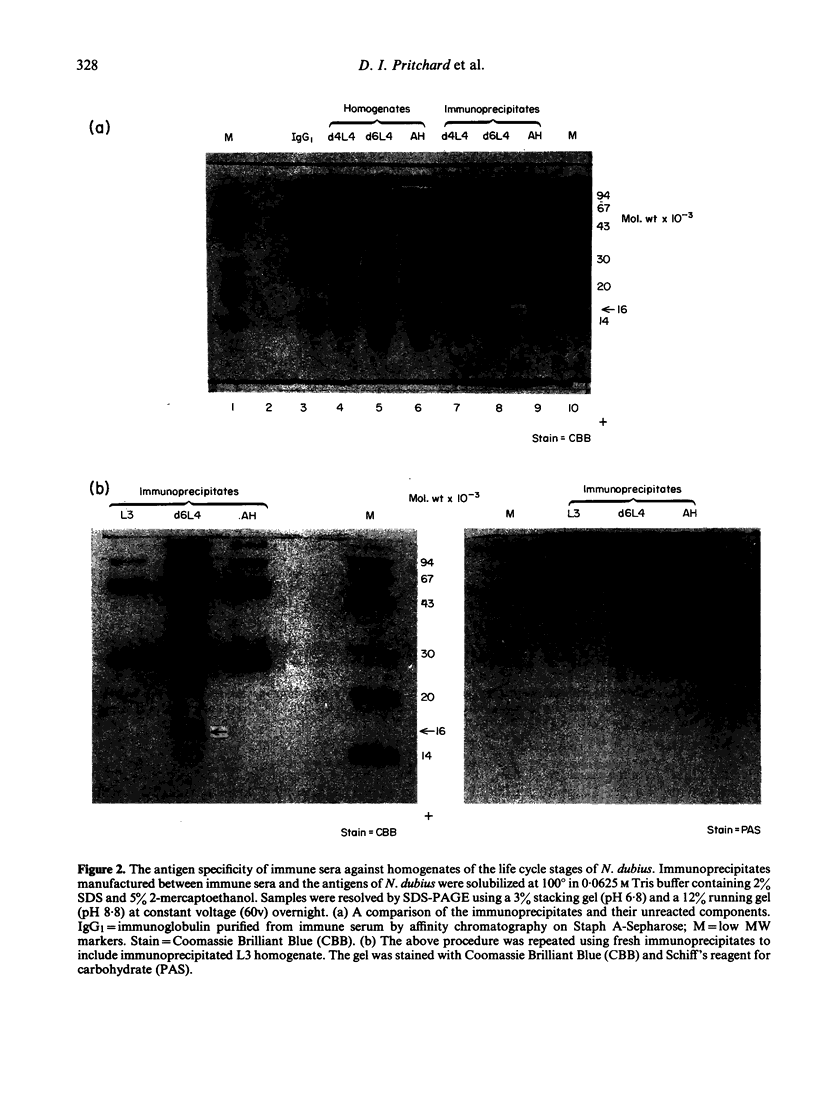
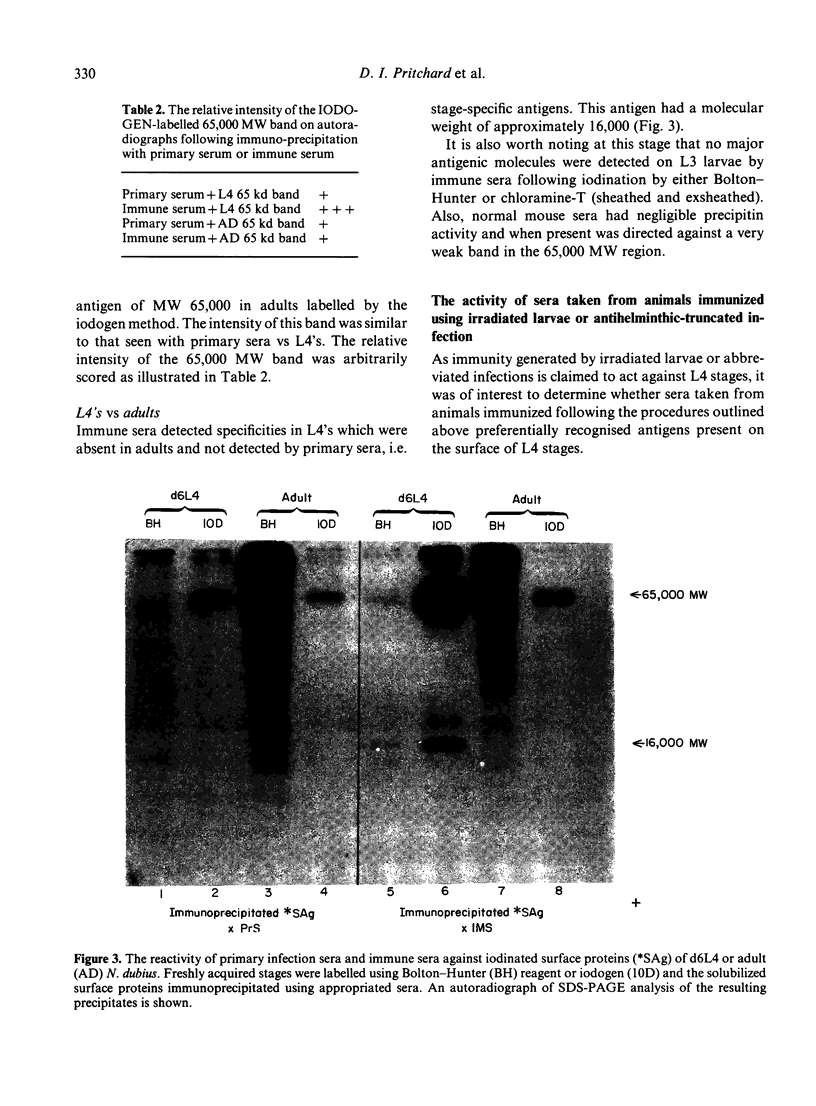
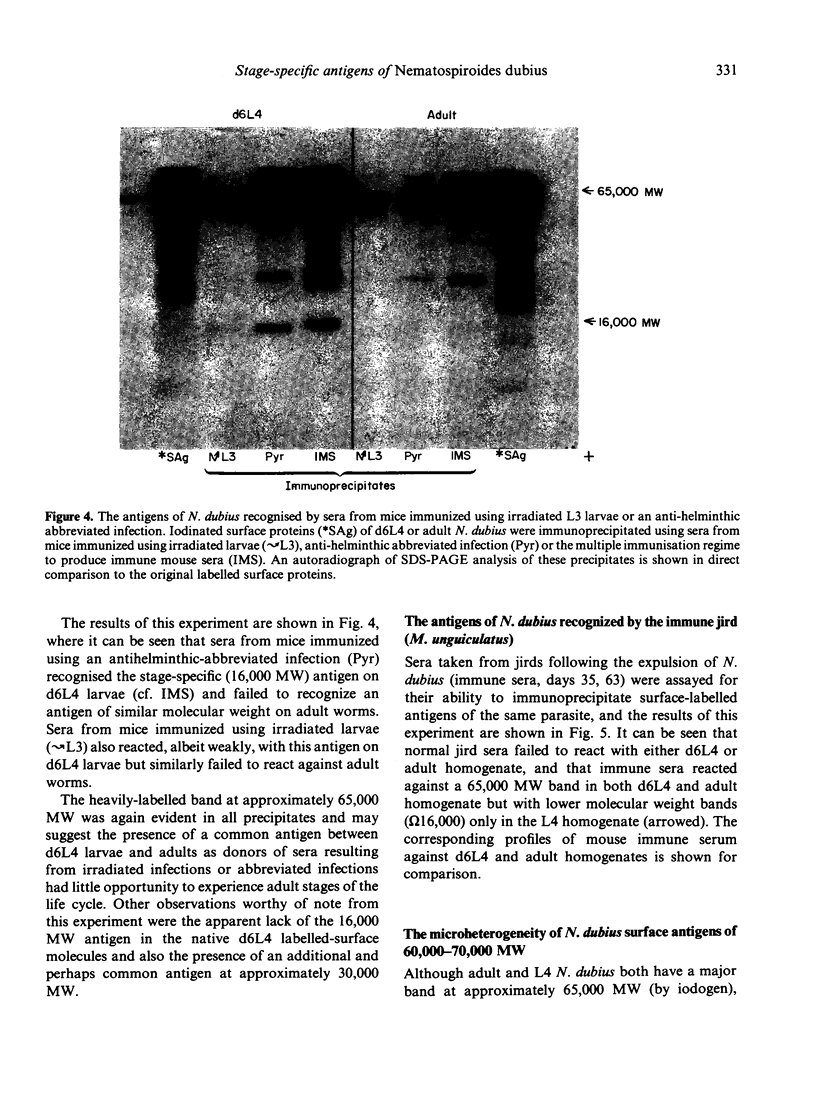
The results presented here demonstrate conclusively for the first time that Nematospiroides dubius exhibits stage specificity in the expression of cuticular antigens, and infer that the stage specific antigens demonstrable on L4 stages 6 days following infection account for the immunity generated by abbreviated infection regimes. L4 stages 6 days following infection appeared to possess a stage-specific surface molecule (MW 16,000) which either disappeared on maturity to adults or somehow became unavailable to the surface probes used in this study. A molecule of similar molecular size was recognized by sera raised in mice immunized either by the administration of irradiated larvae or by using an antihelminthic-abbreviated infection and also by sera from jirds which had recently expelled N. dubius. The immune derivation of these sera supports the hypothesis that the molecule having a MW of 16,000 is functionally immunogenic. A monoclonal antibody (29-1-D1) was used to demonstrate a further stage specific molecule (MW 65,000), this time on the surface of adult N. dubius. The application of biochemical techniques suggested that further heterogeneity existed in surface molecules resolving between 60,000-70,000 on SDS-PAGE and a hypothesis was formulated in an attempt to explain this phenomenon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett A., Ball P. A. The immune response of the mouse to larvae and adults of Nematospiroides dubius. Int J Parasitol. 1974 Oct;4(5):463–470. doi: 10.1016/0020-7519(74)90062-9. [DOI] [PubMed] [Google Scholar]
- Behnke J. M., Hannah J., Pritchard D. I. Nematospiroides dubius in the mouse: evidence that adult worms depress the expression of homologous immunity. Parasite Immunol. 1983 Jul;5(4):397–408. doi: 10.1111/j.1365-3024.1983.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Behnke J. M., Parish H. A. Expulsion of Nematospiroides dubius from the intestine of mice treated with immune serum. Parasite Immunol. 1979 Spring;1(1):13–26. doi: 10.1111/j.1365-3024.1979.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hagan P., Behnke J. M., Parish H. A. Stimulation of immunity to Nematospiroides dubius in mice using larvae attenuated by cobalt 60 irradiation. Parasite Immunol. 1981 Summer;3(2):149–156. doi: 10.1111/j.1365-3024.1981.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Hannah J., Behnke J. M. Nematospiroides dubius in the jird, Meriones unguiculatus: factors affecting the course of a primary infection. J Helminthol. 1982 Dec;56(4):329–338. doi: 10.1017/s0022149x00034738. [DOI] [PubMed] [Google Scholar]
- Lueker D. C., Helper D. I. Differences in immunity to Nematospiroides dubius in inbred and outbred mice. J Parasitol. 1975 Feb;61(1):158–159. [PubMed] [Google Scholar]
- Maizels R. M., Philipp M., Ogilvie B. M. Molecules on the surface of parasitic nematodes as probes of the immune response in infection. Immunol Rev. 1982;61:109–136. doi: 10.1111/j.1600-065x.1982.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Philipp M., Ogilvie B. M. Characterization of surface antigens of Trichinella spiralis infective larvae. Parasite Immunol. 1981 Winter;3(4):339–352. doi: 10.1111/j.1365-3024.1981.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Philipp M., Parkhouse R. M., Ogilvie B. M. Changing proteins on the surface of a parasitic nematode. Nature. 1980 Oct 9;287(5782):538–540. doi: 10.1038/287538a0. [DOI] [PubMed] [Google Scholar]
- Pritchard D. I., Ali N. M., Behnke J. M. Analysis of the mechanism of immunodepression following heterologous antigenic stimulation during concurrent infection with Nematospiroides dubius. Immunology. 1984 Apr;51(4):633–642. [PMC free article] [PubMed] [Google Scholar]
- Pritchard D. I., Williams D. J., Behnke J. M., Lee T. D. The role of IgG1 hypergammaglobulinaemia in immunity to the gastrointestinal nematode Nematospiroides dubius. The immunochemical purification, antigen-specificity and in vivo anti-parasite effect of IgG1 from immune serum. Immunology. 1983 Jun;49(2):353–365. [PMC free article] [PubMed] [Google Scholar]
- Shimp R. G., Crandall R. B., Crandall C. A. Heligmosomoides polygyrus (=Nematospiroides dubius): suppression of antibody response to orally administered sheep erythrocytes in infected mice. Exp Parasitol. 1975 Oct;38(2):257–269. doi: 10.1016/0014-4894(75)90028-4. [DOI] [PubMed] [Google Scholar]