Abstract
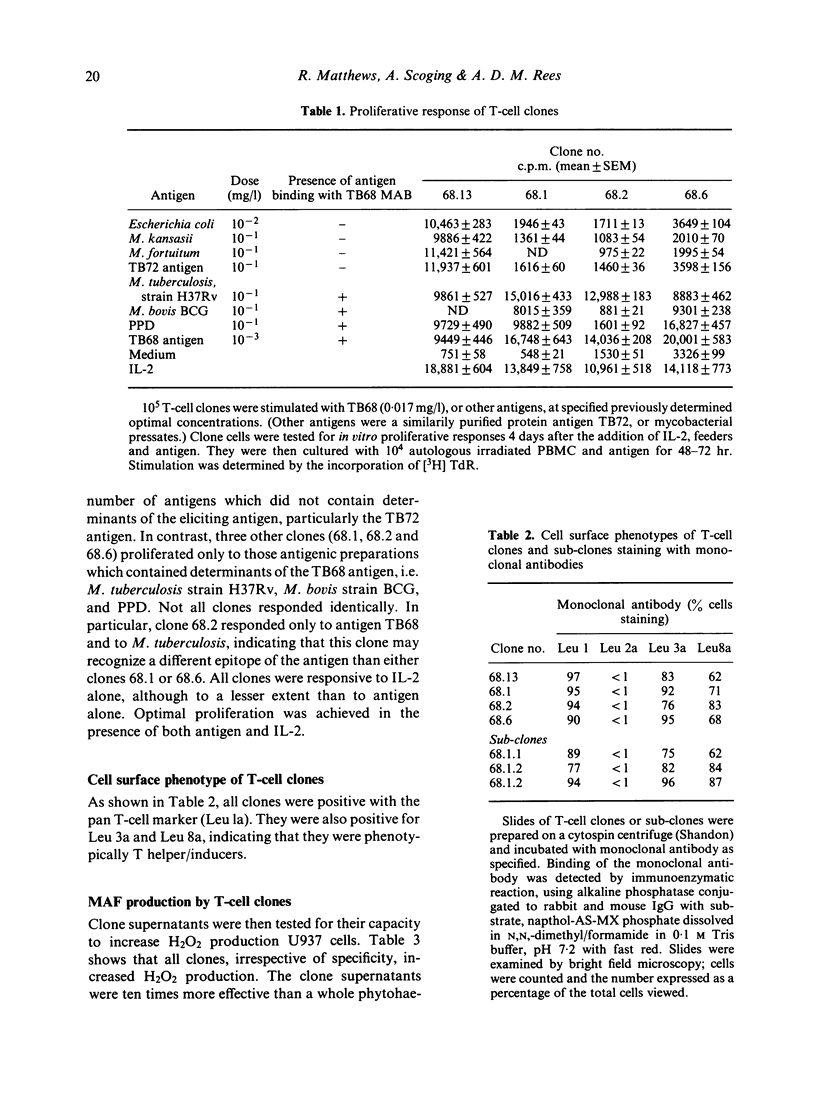
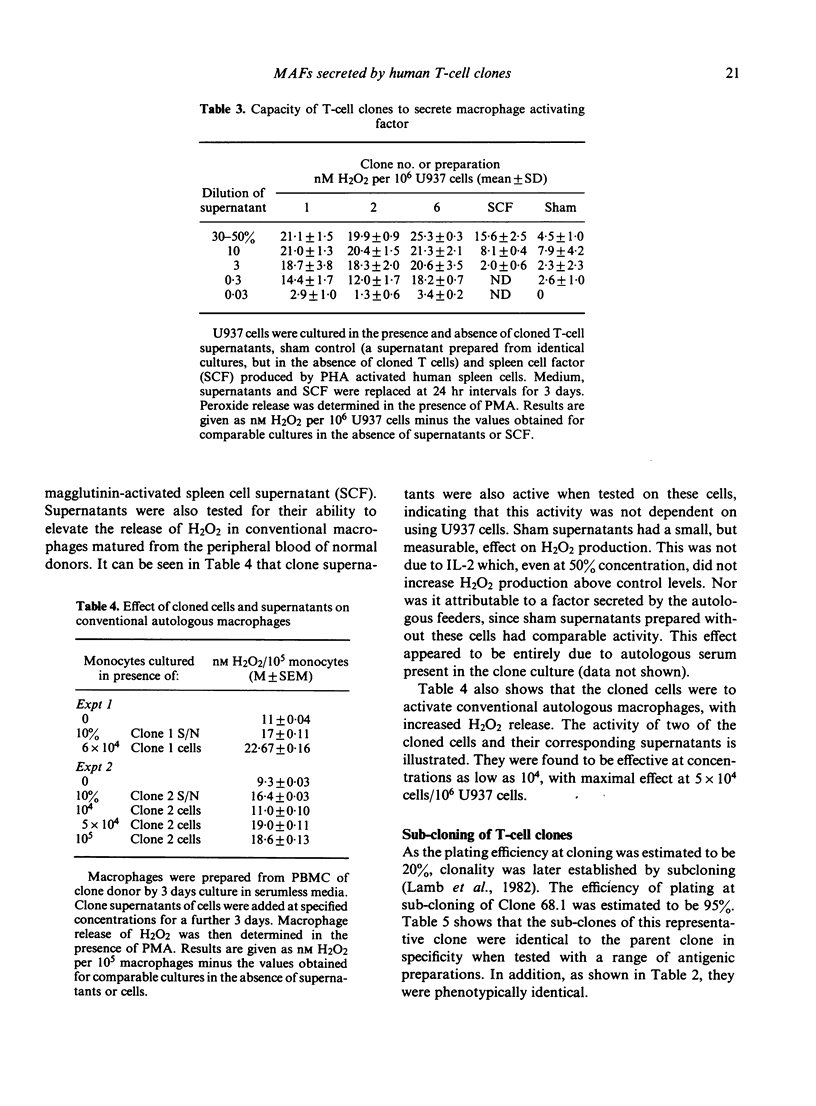
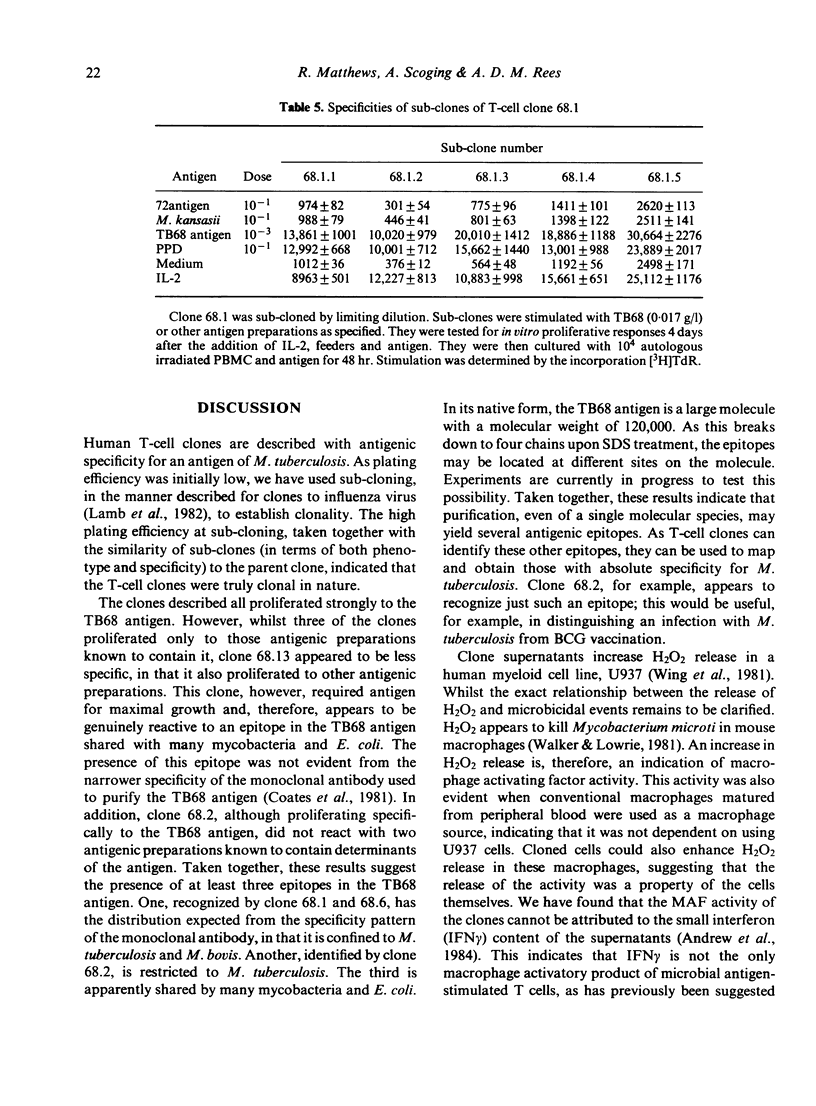
Macrophage activating factor (MAF) is produced by antigen-stimulated lymphocytes and activates macrophages for antimicrobial function. The capacity of individual microbial antigens to evoke and regulate this response has been explored using an affinity purified antigen (TB68) of Mycobacterium tuberculosis in combination with T-cell cloning. Four helper/inducer clones are described which responded strongly to this antigen. Three were specific, proliferating only to TB68 antigen and antigenic preparations containing this antigen. However, one of these clones (68.1) did not proliferate to BCG and PPD which contained the TB68 antigen. In addition, another clone, 68.13, also proliferated to other antigenic preparations which did not contain the TB68 antigen. Taken together, these data indicate the presence of several epitopes in the affinity-purified TB68 antigen. All the clones produced MAF, which enhanced H2O2 production in U937 cell lines and conventional macrophages matured from monocytes. Thus, T-cell clones proliferating to a mycobacterial antigen constitutively secrete lymphokines that activate macrophages to antimicrobial immunity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Crowle A. J., May M. Preliminary demonstration of human tuberculoimmunity in vitro. Infect Immun. 1981 Jan;31(1):453–464. doi: 10.1128/iai.31.1.453-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby P. A., Kansas G. S., Xian C. Y., Evans R. L., Engleman E. G. Dissection of immunoregulatory subpopulations of T lymphocytes within the helper and suppressor sublineages in man. J Immunol. 1982 Nov;129(5):1997–2000. [PubMed] [Google Scholar]
- Gemsa D., Debatin K. M., Kramer W., Kubelka C., Deimann W., Kees U., Krammer P. H. Macrophage-activating factors from different T cell clones induce distinct macrophage functions. J Immunol. 1983 Aug;131(2):833–844. [PubMed] [Google Scholar]
- Godal T., Rees R. J., Lamvik J. O. Lymphocyte-mediated modification of blood-derived macrophage function in vitro; inhibition of growth of intracellular mycobacteria with lymphokines. Clin Exp Immunol. 1971 Apr;8(4):625–637. [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Andrew P. W., Aber V. R., Lowrie D. B. Hydrogen peroxide and superoxide release by alveolar macrophages from normal and BCG-vaccinated guinea-pigs after intravenous challenge with Mycobacterium tuberculosis. Br J Exp Pathol. 1981 Aug;62(4):419–428. [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Johnson A. H., Hartzman R. J., Woody J. N. Antigen-specific human T lymphocyte clones: induction, antigen specificity, and MHC restriction of influenza virus-immune clones. J Immunol. 1982 Jan;128(1):233–238. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir D. J., Ghosh A. K., Abdulaziz Z., Knight P. M., Mason D. Y. Immunoenzymatic staining of haematological samples with monoclonal antibodies. Br J Haematol. 1983 Nov;55(3):395–410. doi: 10.1111/j.1365-2141.1983.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Leonard E. J., Meltzer M. S. Macrophages in resistance to rickettsial infections: characterization of lymphokines that induce rickettsiacidal activity in macrophages. J Immunol. 1981 Jan;126(1):204–207. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Nacy C. A., Meltzer M. S., Williams N., Nakoinz I., Leonard E. J. Colony-stimulating factors and regulation of macrophage tumoricidal and microbicidal activities. Cell Immunol. 1983 Feb 15;76(1):10–21. doi: 10.1016/0008-8749(83)90343-x. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Koren H. S., Fischer D. G., Kelley V. Stimulation of a human macrophage-like cell line (U-937) to inhibit multiplication of an intracellular pathogen. J Reticuloendothel Soc. 1981 Apr;29(4):321–328. [PubMed] [Google Scholar]


