Abstract
The fate of allogeneic lymphocytes (AO or DA) transferred to non-immune PVG recipients was studied in the light of previous evidence (Heslop & McNeilage, 1983; Rolstad & Ford, 1983) that allogeneic lymphocytes can be rapidly destroyed in certain strain combinations of rats and mice by a mechanism that is distinct from either T-cell mediated immunity or an alloantibody response. AO lymphocytes injected into PVG recipients were discriminated from syngeneic lymphocytes within 15-30 min of i.v. injection, as testified by the excess release of 51Cr into the lymph plasma of the recipient. The following experiments were intended to distinguish between natural antibody and natural killer (NK) cells as the mechanism responsible for the allogeneic lymphocyte cytotoxicity (ALC) displayed by PVG rats. Nude rats treated from birth with anti-mu chain serum and shown to be lacking B and T lymphocytes, as well as being profoundly deficient in immunoglobulin, displayed more aggressive ALC than did control nude rats which, in turn, showed stronger ALC than did euthymic rats. Serum from PVG nude rats exerted no inhibitory or destructive effect on allogeneic lymphocytes in an antibody-dependent cellular cytotoxicity system, an assay of graft-versus-host activity, or when injected into 3-4-week-old PVG rats which had not yet developed ALC. Treatment of nude rats with anti-asialo GM 1 antiserum depressed ALC and NK activity in parallel, thus adding to a wide range of circumstances in which ALC and NK activity are closely correlated. In conclusion, ALC is implemented by a non-adaptive, cell-mediated mechanism independent of immunoglobulin, but the precise identity of the effector cell in the recipients' lymphatic tissues remains to be settled.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainbridge D. R. Elimination of allogeneic lymphocytes by mice. Immunol Rev. 1983;73:5–34. doi: 10.1111/j.1600-065x.1983.tb01076.x. [DOI] [PubMed] [Google Scholar]
- Bazin H., Platteau B., Beckers A., Pauwels R. Differential effect of neonatal injections of anti-mu or anti-delta antibodies on the synthesis of IgM, IgD, IgE, IgA, IgG1, IgG2a, IgG2b, and IgG2c immunoglobulin classes. J Immunol. 1978 Nov;121(5):2083–2087. [PubMed] [Google Scholar]
- Bloom B. R. Natural killers to rescue immune surveillance? Nature. 1982 Nov 18;300(5889):214–215. doi: 10.1038/300214a0. [DOI] [PubMed] [Google Scholar]
- Bloom E. T., Babbitt J. T. Rapid kinetics of lysis in human natural cell-mediated cytotoxicity: some implications. Cell Immunol. 1983 Jul 1;79(1):197–206. doi: 10.1016/0008-8749(83)90062-x. [DOI] [PubMed] [Google Scholar]
- Chassoux D., Kolb J. P., Bazin H., MacLennan I. C. Antibody-dependent cellular cytotoxicity (K) and natural killing (NK) in B-suppressed germ-free nude rats. Immunology. 1983 Nov;50(3):327–334. [PMC free article] [PubMed] [Google Scholar]
- Ford W. L., Rolstad B., Fossum S., Hunt S. V., Smith M. E., Sparshott S. M. The stimulus to host cell proliferation in graft-versus-host reactions. Scand J Immunol. 1981 Dec;14(6):705–713. doi: 10.1111/j.1365-3083.1981.tb00613.x. [DOI] [PubMed] [Google Scholar]
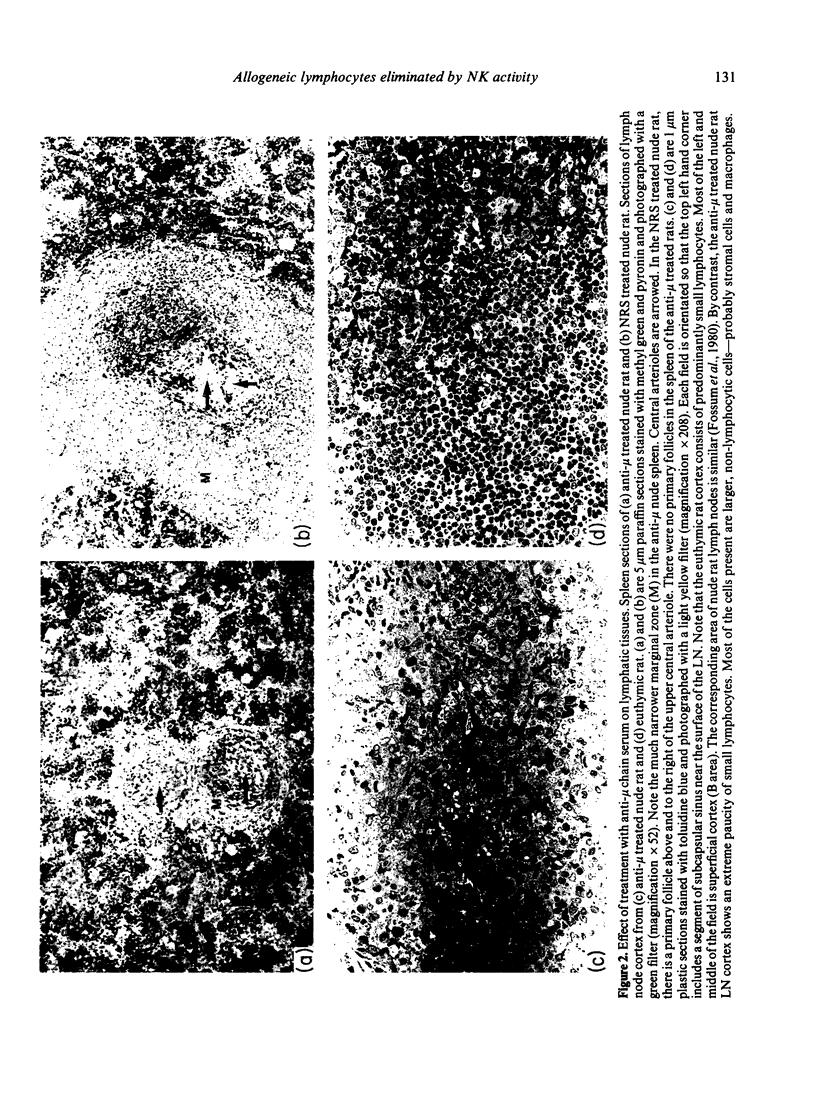
- Fossum S., Smith M. E., Bell E. B., Ford W. L. The architecture of rat lymph nodes. III. The lymph nodes and lymph-borne cells of the congenitally athymic nude rat (rnu). Scand J Immunol. 1980;12(5):421–432. doi: 10.1111/j.1365-3083.1980.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Heslop B. F., Hardy B. E. In vivo damage to circulating lymphoid cells by alloimmune serum in rats. Transplantation. 1972 Apr;13(4):364–371. doi: 10.1097/00007890-197204000-00003. [DOI] [PubMed] [Google Scholar]
- Heslop B. F., McNeilage L. J. Natural cytotoxicity: early killing of allogeneic lymphocytes in rats. Immunol Rev. 1983;73:35–51. doi: 10.1111/j.1600-065x.1983.tb01077.x. [DOI] [PubMed] [Google Scholar]
- Heslop B. F., McNeilage L. J., Sengupta S. Allogeneic lymphocyte cytotoxicity in rats: the effects of various pharmacological agents. Immunology. 1984 Sep;53(1):43–53. [PMC free article] [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Hochman P. S., Haller O., Shearer G. M., Wigzell H., Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur J Immunol. 1977 Sep;7(9):655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- Kimber I., Bakács T., Moore M. Regulation of natural and antibody-dependent cellular cytotoxicity by staphylococcal enterotoxin A. Clin Exp Immunol. 1983 Oct;54(1):39–48. [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. M., Ayala E. Active and passive immunity against Pseudomonas aeruginosa with a ribosomal vaccine and antiserum in C3H/HeJ mice. J Immunol. 1983 Jul;131(1):1–3. [PubMed] [Google Scholar]
- Lotzová E., Savary C. A., Pollack S. B. Prevention of rejection of allogeneic bone marrow transplants by NK 1.1 antiserum. Transplantation. 1983 May;35(5):490–494. doi: 10.1097/00007890-198305000-00019. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. Antibody in the induction and inhibition of lymphocyte cytotoxicity. Transplant Rev. 1972;13:67–90. doi: 10.1111/j.1600-065x.1972.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McNeilage L. J., Heslop B. F. Lymphoycte homing in syngeneic and unsensitized MHC compatible allogeneic hosts. I. Evidence for both syngeneic self-recognition and early killing of allogeneic cells. Cell Immunol. 1980 Mar 1;50(1):58–70. doi: 10.1016/0008-8749(80)90006-4. [DOI] [PubMed] [Google Scholar]
- McNeilage L. J., Heslop B. F. Natural cytotoxicity in rats: radiation-induced changes in the early killing of allogeneic cells. Cell Immunol. 1983 Jun;78(2):206–216. doi: 10.1016/0008-8749(83)90275-7. [DOI] [PubMed] [Google Scholar]
- Reynolds C. W., Timonen T. T., Holden H. T., Hansen C. T., Herberman R. B. Natural killer cell activity in the rat. Analysis of effector cell morphology and effects of interferon on natural killer cell function in the athymic (nude) rat. Eur J Immunol. 1982 Jul;12(7):577–582. doi: 10.1002/eji.1830120709. [DOI] [PubMed] [Google Scholar]
- Rolstad B., Ford W. L. The rapid elimination of allogeneic lymphocytes: relationship to established mechanisms of immunity and to lymphocyte traffic. Immunol Rev. 1983;73:87–113. doi: 10.1111/j.1600-065x.1983.tb01080.x. [DOI] [PubMed] [Google Scholar]
- Rolstad B. The influence of strong transplantation antigens (Ag-B) on lymphocyte migration in vivo. Cell Immunol. 1979 Jul;45(2):389–397. doi: 10.1016/0008-8749(79)90399-x. [DOI] [PubMed] [Google Scholar]
- Rolstad B., Williams A. F., Ford W. L. The alloantibody response to a strong transplantation antigen (Ag-B). Quantitative aspects and thymus dependence of the response. Transplantation. 1974 Apr;17(4):416–423. doi: 10.1097/00007890-197404000-00013. [DOI] [PubMed] [Google Scholar]
- Rooney C. M., Munro A. J. NK cells can recognize asialylated autologous lymphocytes and ABO-mismatched lymphocytes. Immunology. 1984 Jan;51(1):193–199. [PMC free article] [PubMed] [Google Scholar]
- Tilney N. L. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971 Sep;109(Pt 3):369–383. [PMC free article] [PubMed] [Google Scholar]
- Tønnesen B., Rolstad B. In vivo elimination of allogeneic lymphocytes in normal and T-cell-deficient rats. Elimination does not require T cells. Scand J Immunol. 1983 Apr;17(4):303–312. doi: 10.1111/j.1365-3083.1983.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Viklický V., Pavlík L. The reduced localization of iv-injected lymphocytes into the lymph nodes, lungs, and small intestine of allogeneic hosts is a consequence of cell destruction. Cell Immunol. 1981 Oct;64(1):13–19. doi: 10.1016/0008-8749(81)90453-6. [DOI] [PubMed] [Google Scholar]



