Abstract
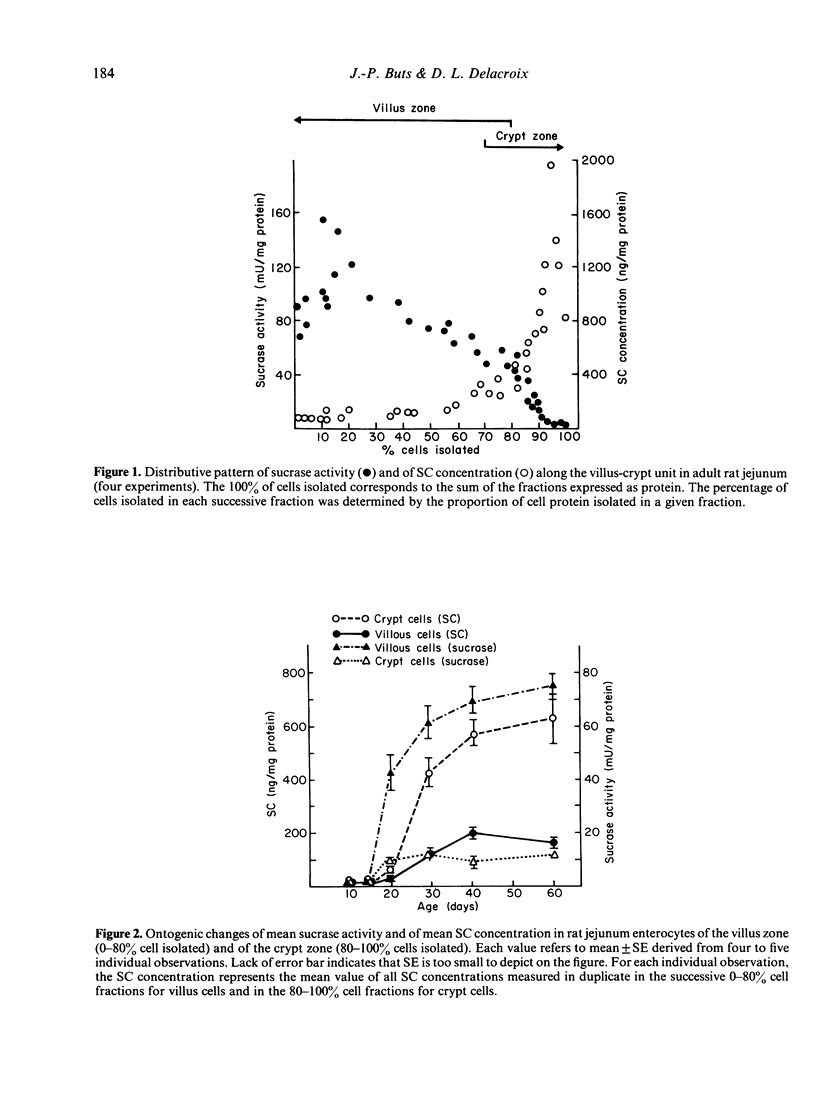
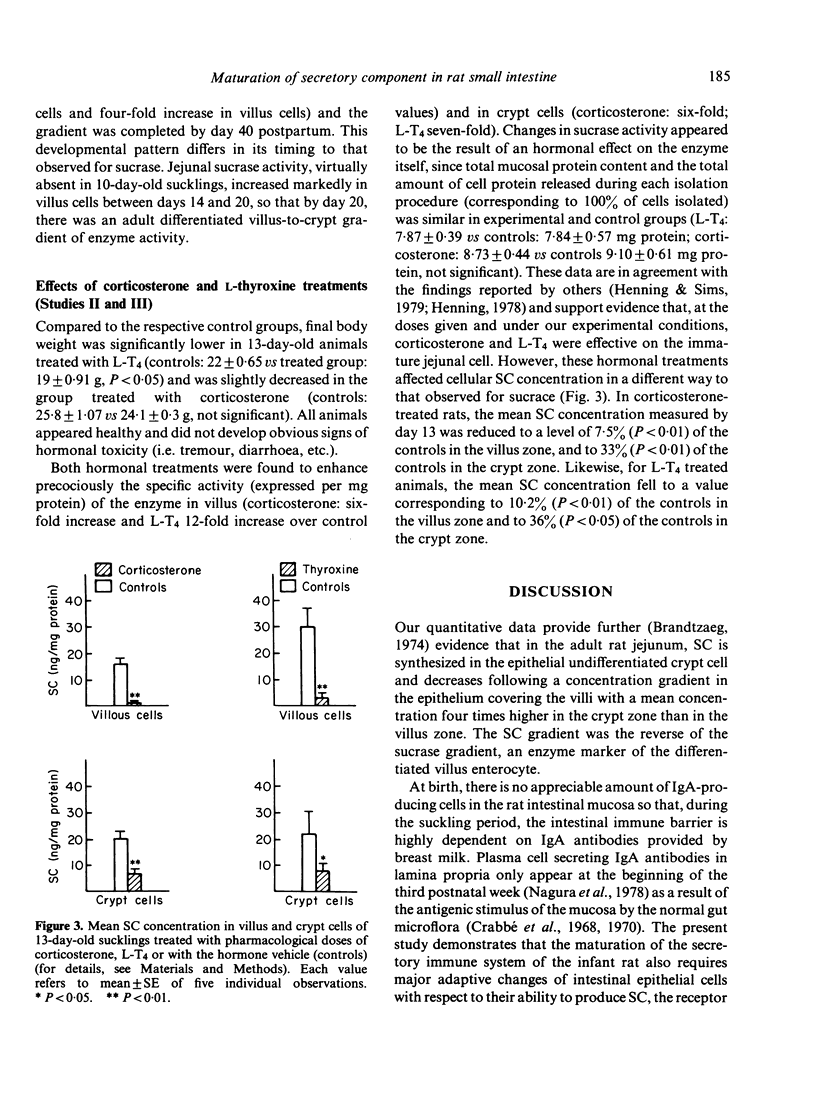
In order to determine whether the rat small intestine exhibits quantitative changes in the synthesis of the secretory component (SC) during growth, epithelial villus and crypt cells were isolated from jejunal segments at intervals after birth up to adulthood. SC concentration was measured in each cell fraction by immunoradiometric assay and compared to sucrase activity, an enzyme marker of the differentiated villus enterocyte. The following results were observed. (i) Adult rats showed a characteristic decreasing concentration gradient of SC from the crypts (mean concentration in crypt cells: 636 +/- 173 ng/mg protein) to the villus tip (mean concentration in villus cells: 152 +/- 17 ng/mg protein). This gradient was the reverse of that found for sucrase activity. (ii) In young sucklings (10 days old), SC was virtually absent in both villus and crypt cells, but its concentration progressively increased in weanling rats and reached adult levels by day 40 postpartum. (iii) The crypt to villus cell gradient of SC, absent in sucklings up to day 20, developed during the fourth postnatal week. (iv) Treatment of 10-day-old suckling pups with pharmacological doses of either corticosterone or L-thyroxine for 3 consecutive days failed to induce the precocious synthesis of SC by jejunal enterocytes, but produced significant (P less than 0.01) decreases in concentration. Under the same conditions, sucrase activity was markedly enhanced. In conclusion, major changes in the ability of the immature crypt cell to produce the specific receptor for transepithelial transport of polymeric immunoglobulins occur during the fourth week of rat life. The initiation of this ontogenic process is not triggered by the dietary and hormonal changes known to control the maturation of other functions linked to the differenciated villus cell, such as sucrase activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components: differential localization of free and bound SC in secretory epithelial cells. J Immunol. 1974 Apr;112(4):1553–1559. [PubMed] [Google Scholar]
- Brandtzaeg P. Transport models for secretory IgA and secretory IgM. Clin Exp Immunol. 1981 May;44(2):221–232. [PMC free article] [PubMed] [Google Scholar]
- Brown W. R., Isobe K., Nakane P. K., Pacini B. Studies on translocation of immunoglobulins across intestinal epithelium. IV. Evidence for binding of IgA and IgM to secretory component in intestinal epithelium. Gastroenterology. 1977 Dec;73(6):1333–1339. [PubMed] [Google Scholar]
- Buts J. P., De Meyer R. Intestinal development in the suckling rat: effects of weaning, diet composition, and glucocorticoids on thymidine kinase activity and DNA synthesis. Pediatr Res. 1984 Feb;18(2):145–150. doi: 10.1203/00006450-198402000-00006. [DOI] [PubMed] [Google Scholar]
- Buts J. P., De Meyer R. Postnatal proximodistal development of the small bowel mucosal mass in growing rats. Biol Neonate. 1981;40(1-2):62–69. doi: 10.1159/000241473. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Bazin H., Eyssen H., Heremans J. F. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Appl Immunol. 1968;34(4):362–375. doi: 10.1159/000230130. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Nash D. R., Bazin H., Eyssen H., Heremans J. F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Invest. 1970 May;22(5):448–457. [PubMed] [Google Scholar]
- Crago S. S., Kulhavy R., Prince S. J., Mestecky J. Secretory component of epithelial cells is a surface receptor for polymeric immunoglobulins. J Exp Med. 1978 Jun 1;147(6):1832–1837. doi: 10.1084/jem.147.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Vaerman J. P. A solid phase, direct competition, radioimmunoassay for quantitation of secretory IgA in human serum. J Immunol Methods. 1981;40(3):345–358. doi: 10.1016/0022-1759(81)90366-5. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Vaerman J. P. Secretory component (SC): preferential binding to heavy (greater than 11S) IgA polymers and IgM in serum, in contrast to predominance of 11S and free SC forms in secretions. Clin Exp Immunol. 1982 Sep;49(3):717–724. [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Monoclonal antibodies to sucrase/isomaltase: probes for the study of postnatal development and biogenesis of the intestinal microvillus membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6629–6633. doi: 10.1073/pnas.77.11.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning S. J., Sims J. M. Delineation of the glucocorticoid-sensitive period of intestinal development in the rat. Endocrinology. 1979 Apr;104(4):1158–1163. doi: 10.1210/endo-104-4-1158. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Messer M., Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966 Mar;14(3):376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Montgomery R. K., Sybicki M. A., Grand R. J. Autonomous biochemical and morphological differentiation in fetal rat intestine transplanted at 17 and 20 days of gestation. Dev Biol. 1981 Oct 15;87(1):76–84. doi: 10.1016/0012-1606(81)90062-2. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Blobel G. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J Biol Chem. 1982 Oct 10;257(19):11816–11821. [PubMed] [Google Scholar]
- Mostov K. E., Kraehenbuhl J. P., Blobel G. Receptor-mediated transcellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7257–7261. doi: 10.1073/pnas.77.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagura H., Nakane P. K., Brown W. R. Breast milk IgA binds to jejunal epithelium in suckling rats. J Immunol. 1978 Apr;120(4):1333–1339. [PubMed] [Google Scholar]
- Nagura H., Nakane P. K., Brown W. R. Secretory component in immmunoglobulin deficiency: and immunoelectron microscopic study of intestinal epithelium. Scand J Immunol. 1980;12(4):359–363. doi: 10.1111/j.1365-3083.1980.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Raul F., Simon P., Kedinger M., Haffen K. Intestinal enzymes activities in isolated villus and crypt cells during postnatal development of the rat. Cell Tissue Res. 1977 Jan 12;176(2):167–178. doi: 10.1007/BF00229460. [DOI] [PubMed] [Google Scholar]
- Samel M. Thyroid function during postnatal development in the rat. Gen Comp Endocrinol. 1968 Apr;10(2):229–234. doi: 10.1016/0016-6480(68)90029-4. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]


