Abstract
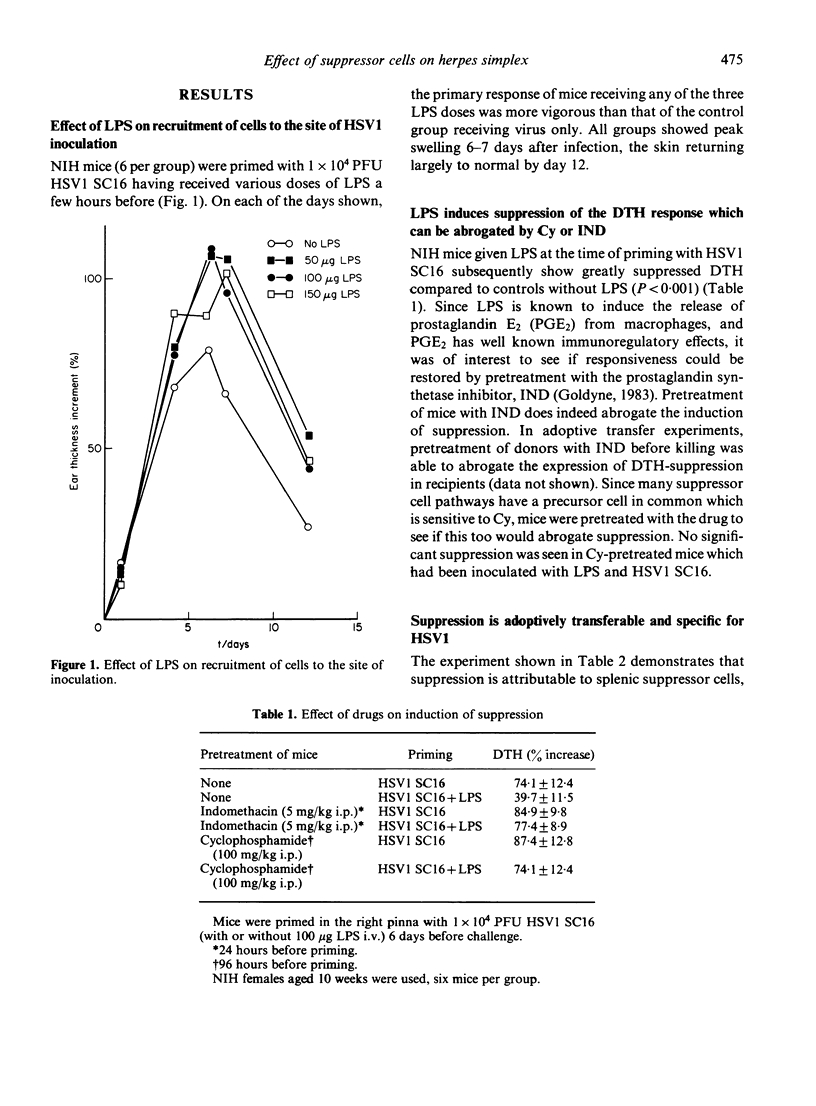
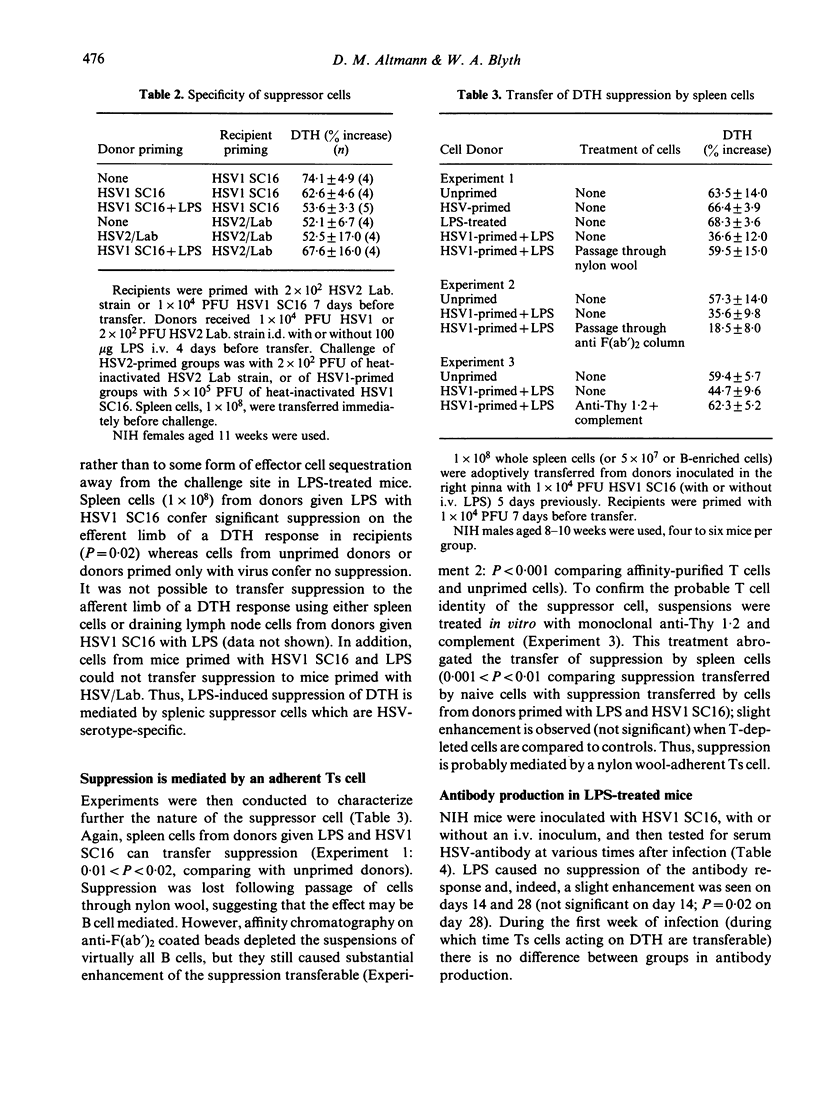
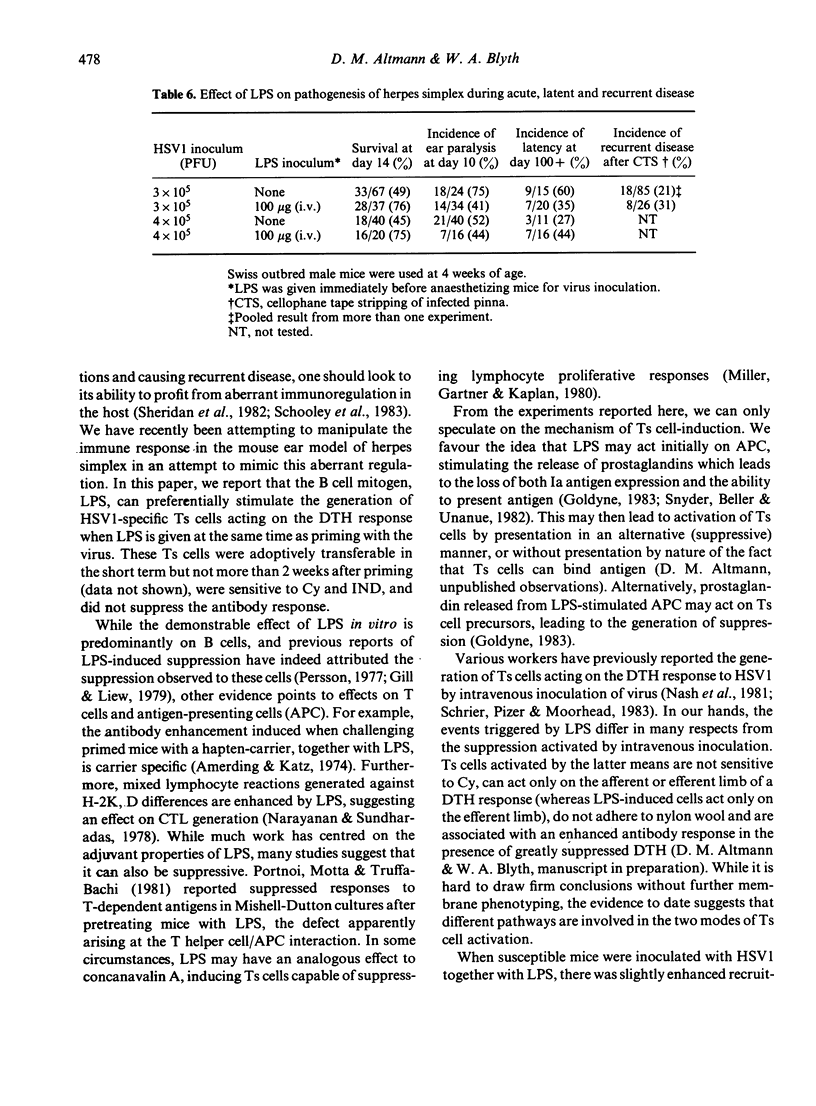
Treatment of mice with LPS at the time of priming with herpes simplex virus type 1 (HSV1) causes the preferential activation of virus-specific T suppressor (Ts) cells. These Ts cells can transfer suppression to the efferent limb of a DTH response. Priming under these conditions is associated with enhanced cell-recruitment to the inoculation site, but had no effect on virus clearance. The induction of suppression was abrogated by pretreatment of mice with cyclophosphamide or indomethacin. LPS had no effect on the antibody response to HSV1 during acute infection, although treated mice showed a raised antibody titre one month after inoculation. Susceptible mice inoculated with HSV1 and given LPS showed protection, both from lethal herpes encephalitis and from demyelination within the CNS as reflected by ear paralysis. These results imply that, during some stages of acute infection, T cell effector mechanisms may themselves mediate tissue damage. At such times, Ts cells may perform a beneficial role leading to a reduction in pathology.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J Exp Med. 1974 Jan 1;139(1):24–43. doi: 10.1084/jem.139.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darville J. M., Blyth W. A. Neutralising antibody in mice with primary and recurrent herpes simplex virus infection. Arch Virol. 1982;71(4):303–310. doi: 10.1007/BF01315060. [DOI] [PubMed] [Google Scholar]
- Gill H. K., Liew F. Y. Regulation of delayed-type hypersensitivity IV. Antigen-specific suppressor cells for delayed-type hypersensitivity induced by lipopolysaccharide and sheep erythrocytes in mice. Eur J Immunol. 1979 Feb;9(2):101–106. doi: 10.1002/eji.1830090202. [DOI] [PubMed] [Google Scholar]
- Harbour D. A., Hill T. J., Blyth W. A. Acute and recurrent herpes simplex in several strains of mice. J Gen Virol. 1981 Jul;55(Pt 1):31–40. doi: 10.1099/0022-1317-55-1-31. [DOI] [PubMed] [Google Scholar]
- Hill T. J., Blyth W. A., Harbour D. A., Berrie E. L., Tullo A. B. Latency and other consequences of infection of the nervous system with herpes simplex virus. Prog Brain Res. 1983;59:173–184. doi: 10.1016/S0079-6123(08)63862-5. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Gartner S., Kaplan H. S. The induction of suppressor T cells by lipopolysaccharide in human peripheral blood lymphocyte cultures in the presence of fetal calf serum. Cell Immunol. 1980 Sep 15;55(1):210–218. doi: 10.1016/0008-8749(80)90152-5. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Hirsch M. S., Kramer J. H., Murphy F. A. Effect of antithymocyte serum on herpesvirus hominis (type 1) infection in adult mice. Proc Soc Exp Biol Med. 1969 Nov;132(2):696–698. doi: 10.3181/00379727-132-34290. [DOI] [PubMed] [Google Scholar]
- Narayanan P. R., Sundharadas G. Differential effects of polyadenylic: polyuridylic acid and lipopolysaccharide on the generation of cytotoxic T lymphocytes. J Exp Med. 1978 May 1;147(5):1355–1362. doi: 10.1084/jem.147.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Ashford N. P. Split T-cell tolerance in herpes simplex virus-infected mice and its implication for anti-viral immunity. Immunology. 1982 Apr;45(4):761–767. [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Phelan J., Gell P. G., Wildy P. Tolerance and immunity in mice infected with herpes simplex virus: studies on the mechanism of tolerance to delayed-type hypersensitivity. Immunology. 1981 Jun;43(2):363–369. [PMC free article] [PubMed] [Google Scholar]
- Persson U. Lipopolysaccharide-induced suppression of the primary immune response to a thymus-dependent antigen. J Immunol. 1977 Mar;118(3):789–796. [PubMed] [Google Scholar]
- Portnoï D., Motta I., Truffa-Bachi P. Immune unresponsiveness of spleen cells from lipopolysaccharide-treated mice to particulate thymus-dependent antigen. I. Evidence for differentiation signal defect. Eur J Immunol. 1981 Feb;11(2):156–158. doi: 10.1002/eji.1830110218. [DOI] [PubMed] [Google Scholar]
- Schooley R. T., Hirsch M. S., Colvin R. B., Cosimi A. B., Tolkoff-Rubin N. E., McCluskey R. T., Burton R. C., Russell P. S., Herrin J. T., Delmonico F. L. Association of herpesvirus infections with T-lymphocyte-subset alterations, glomerulopathy, and opportunistic infections after renal transplantation. N Engl J Med. 1983 Feb 10;308(6):307–313. doi: 10.1056/NEJM198302103080603. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Pizer L. I., Moorhead J. W. Tolerance and suppression of immunity to herpes simplex virus: different presentations of antigens induce different types of suppressor cells. Infect Immun. 1983 May;40(2):514–522. doi: 10.1128/iai.40.2.514-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand F. L. Analysis of immunosuppression generated by the graft-versus-host reaction. I. A suppressor T-cell component studied in vivo. Immunology. 1975 Dec;29(6):953–965. [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. F., Donnenberg A. D., Aurelian L., Elpern D. J. Immunity to herpes simplex virus type 2. IV. Impaired lymphokine production during recrudescence correlates with an imbalance in T lymphocyte subsets. J Immunol. 1982 Jul;129(1):326–331. [PubMed] [Google Scholar]
- Snyder D. S., Beller D. I., Unanue E. R. Prostaglandins modulate macrophage Ia expression. Nature. 1982 Sep 9;299(5879):163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- Townsend J. J., Baringer J. R. Morphology of central nervous system disease in immunosuppressed mice after peripheral herpes simplex virus inoculation. Trigeminal root entry zone. Lab Invest. 1979 Feb;40(2):178–182. [PubMed] [Google Scholar]
- Zawatzky R., Hilfenhaus J., Krichner H. Resistance of nude mice to herpes simplex virus and correlation with in vitro production of interferon. Cell Immunol. 1979 Oct;47(2):424–428. doi: 10.1016/0008-8749(79)90352-6. [DOI] [PubMed] [Google Scholar]


