Abstract
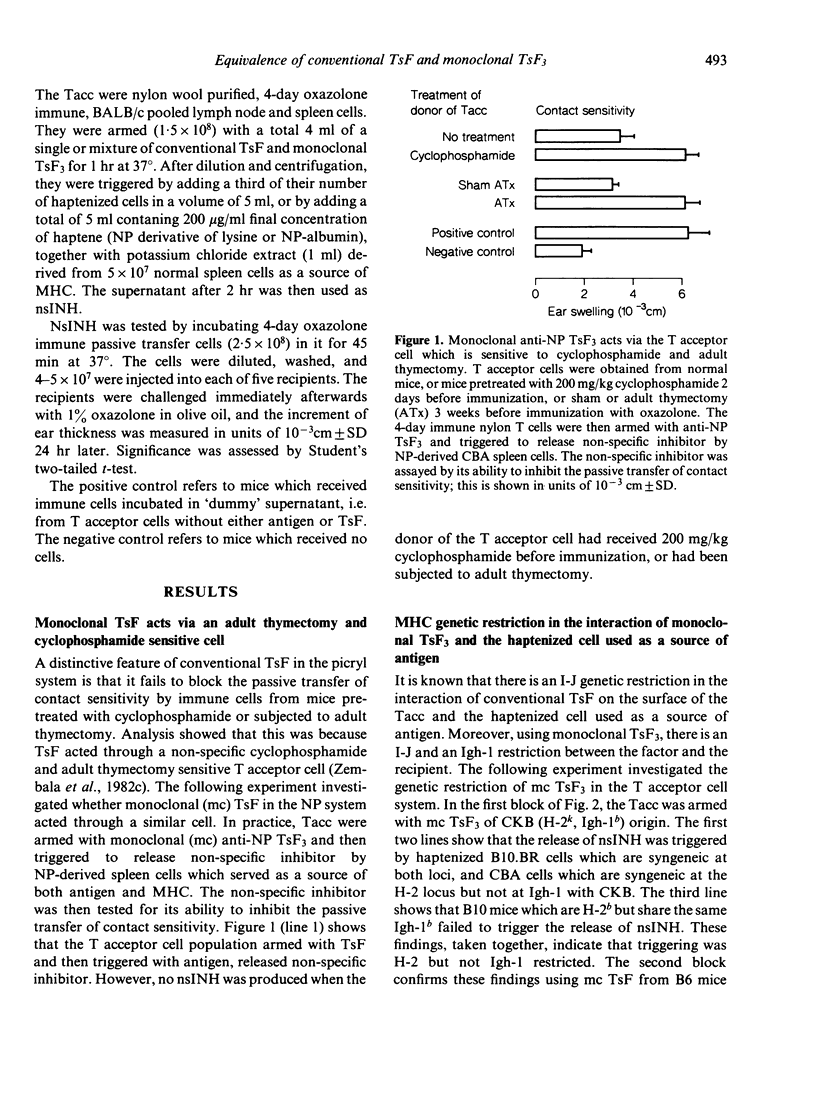
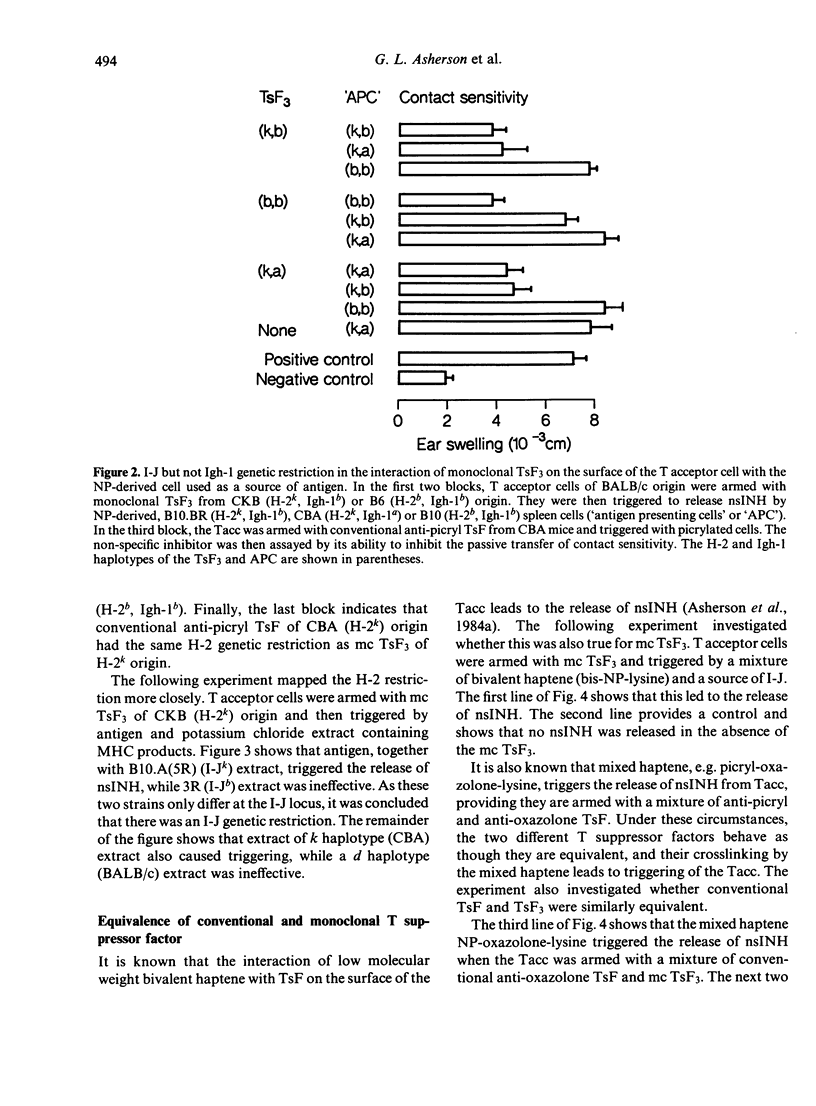
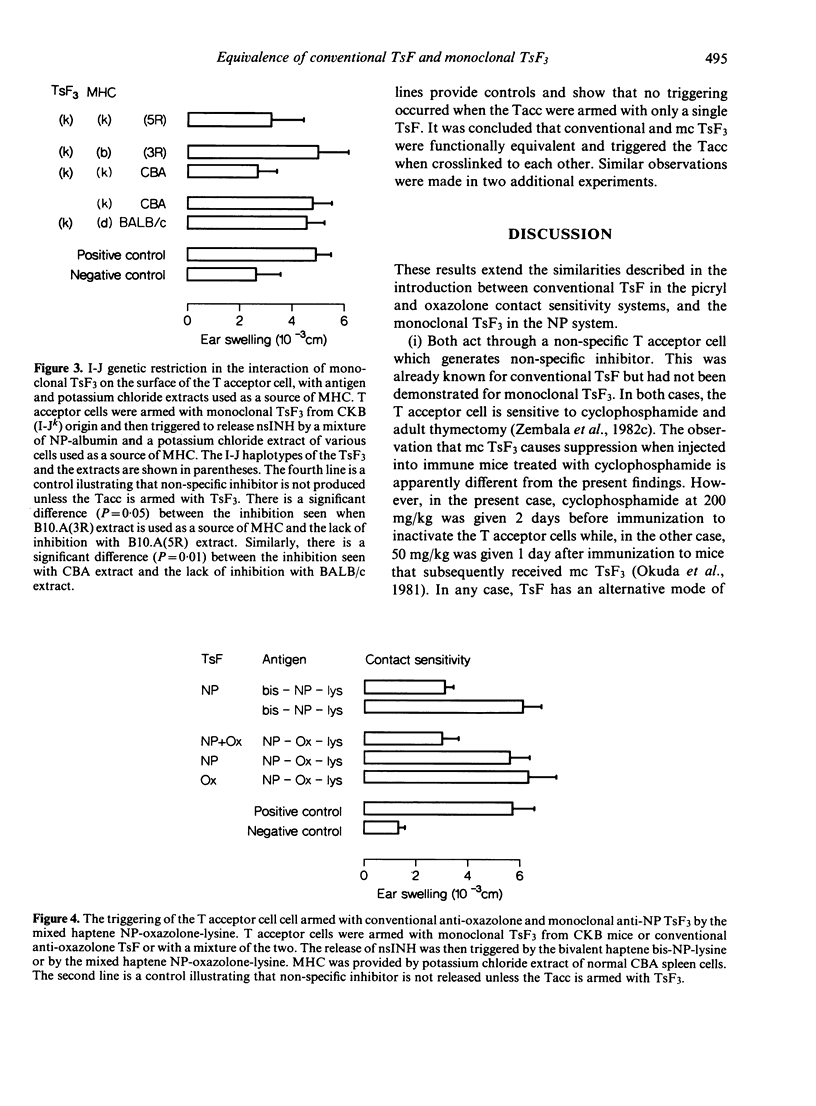
There is considerable confusion over whether the antigen-specific T suppressor factors (TsF) described by different authors are indeed equivalent. This paper investigates whether monoclonal TsF3, obtained from hybridomas derived from mice injected subcutaneously with NP derived spleen cells, is functionally equivalent to the conventional T suppressor factor, produced by mice injected intravenously with chemically reactive, water soluble haptene (picrylsulphonic acid and oxazolone thioglycolic acid). Comparison of monoclonal anti-NP TsF3 with conventional anti-picryl and anti-oxazolone T suppressor factor showed that both armed the non-specific T acceptor cell (Tacc) which was sensitive to cyclophosphamide and adult thymectomy. Moreover, non-specific inhibitor (nsINH) of the transfer of contact sensitivity was released when antigen, together with major histocompatibility complex products (MHC), reacted with conventional or monoclonal TsF on the surface of the non-specific T acceptor cell. The interaction of monoclonal TsF3 with antigen, which led to the release of NsINH, required the presence of MHC and was I-J restricted. However, there was no Igh-1 restriction. The equivalence of conventional anti-picryl and anti-oxazolone TsF has been demonstrated by arming the Tacc with a mixture of these two suppressor factors, and then triggering the release of nsINH with the mixed haptene 'picryl-oxazolone-lysine' which crosslinks separate molecules of TsF. A similar equivalence of conventional anti-oxazolone TsF and monoclonal anti-NP TsF3 was demonstrated using the mixed hapten 'NP-oxazolone-lysine' to trigger the release of nsINH. It was concluded that monoclonal TsF3 and conventional TsF were equivalent, and that both had an indirect mode of action through the non-specific T acceptor cell which led to the production of non-specific inhibitor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Watkins M. C., Zembala M. A., Colizzi V. C. Two-chain structure of T-suppressor factor: antigen-specific T-suppressor factor occurs as a single molecule and as separate antigen-binding and I-J+ parts, both of which are required for biological activity. Cell Immunol. 1984 Jul;86(2):448–459. doi: 10.1016/0008-8749(84)90400-3. [DOI] [PubMed] [Google Scholar]
- Brownstone A., Mitchison N. A., Pitt-Rivers R. Chemical and serological studies with an iodine-containing synthetic immunological determinant 4-hydroxy-3-iodo-5-nitrophenylacetic acid (NIP) and related compounds. Immunology. 1966 May;10(5):465–479. [PMC free article] [PubMed] [Google Scholar]
- Colizzi V., Asherson G. L., James B. M. The role of I-J in the suppressor T-cell circuit which influences the effector stage of contact sensitivity: antigen together with syngeneic I-J region determinants induces and activates T suppressor cells. Immunology. 1983 May;49(1):191–199. [PMC free article] [PubMed] [Google Scholar]
- Malkovský M., Asherson G. L., Chandler P., Colizzi V., Watkins M. C., Zembala M. Nonspecific inhibitor of DNA synthesis elaborated by T acceptor cells. I. Specific hapten- and I-J-driven liberation of an inhibitor of cell proliferation by Lyt-1-2+ cyclophosphamide-sensitive T acceptor cells armed with a product of Lyt-1+2+-specific suppressor cells. J Immunol. 1983 Feb;130(2):785–790. [PubMed] [Google Scholar]
- Minami M., Furusawa S., Dorf M. E. I-J restrictions on the activation and interaction of parental and F1-derived TS3 suppressor cells. J Exp Med. 1982 Aug 1;156(2):465–479. doi: 10.1084/jem.156.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M., Honji N., Dorf M. E. Mechanism responsible for the induction of I-J restriction on TS3 suppressor cells. J Exp Med. 1982 Nov 1;156(5):1502–1515. doi: 10.1084/jem.156.5.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Minami M., Furusawa M., Dorf M. E. Analysis of T cell hybridomas. II. Comparisons among three distinct types of monoclonal suppressor factors. J Exp Med. 1981 Dec 1;154(6):1838–1851. doi: 10.1084/jem.154.6.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Zembala M., Hanczakowski-Rewicka M., Asherson G. L. Nonspecific macrophage suppressor factor: its role in the inhibition of contact sensitivity to picryl chloride by specific T suppressor factor. Eur J Immunol. 1978 Sep;8(9):645–649. doi: 10.1002/eji.1830080908. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Pellegrino M. A., Kahan B. D. Salt extraction of soluble HL-A antigens. Science. 1971 Jun 11;172(3988):1134–1136. doi: 10.1126/science.172.3988.1134. [DOI] [PubMed] [Google Scholar]
- Sherr D. H., Minami M., Okuda K., Dorf M. E. Analysis of T cell hybridomas. III. Distinctions between two types of hapten-specific suppressor factors that affect plaque-forming cell responses. J Exp Med. 1983 Feb 1;157(2):515–529. doi: 10.1084/jem.157.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday M. E., Benacerraf B., Dorf M. E. Hapten-specific T cell responses to 4-hydroxy-3-nitrophenyl acetyl. VIII. Suppressor cell pathways in cutaneous sensitivity responses. J Exp Med. 1981 Apr 1;153(4):811–822. doi: 10.1084/jem.153.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger J. Z., Benacerraf B., Dorf M. E. Hapten-specific T cell responses to 4-hydroxy-3-nitrophenyl acetyl. III. Interaction of effector suppressor T cells is restricted by H-2 and Igh-V genes. J Exp Med. 1980 Jun 1;151(6):1413–1423. doi: 10.1084/jem.151.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembala M. A., Asherson G. L., James B. M., Stein V. E., Watkins M. C. Anti-haptene T suppressor factor acts through an I-J+, Ly1-2+, T acceptor cell that releases a nonspecific inhibitor of the transfer of contact sensitivity when exposed to antigen. J Immunol. 1982 Nov;129(5):1823–1829. [PubMed] [Google Scholar]
- Zembala M., Asherson G. L., Colizzi V. Hapten-specific T suppressor factor recognizes both hapten and I-J region products on haptenized spleen cells. Nature. 1982 Jun 3;297(5865):411–413. doi: 10.1038/297411a0. [DOI] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L., Colizzi V., Watkins M. C. Desensitization in vitro: the role of T-suppressor cells, T-suppressor factor and T-acceptor cells in the inhibition of the passive transfer of contact sensitivity to picryl chloride by exposure to antigen in vitro. Immunology. 1982 Dec;47(4):605–615. [PMC free article] [PubMed] [Google Scholar]


