Abstract
Schizosaccharomyces pombe cells grow from both ends during most of interphase and divide symmetrically into two daughter cells. The pom1 gene, encoding a member of the Dyrk family of protein kinases, has been identified through a mutant showing abnormal cellular morphogenesis. Here we show that Pom1p kinase activity is cell cycle regulated in correlation with the state of cellular symmetry: the activity is high during symmetrical growth and division, but lower when cells grow at just one end. Point mutations in the catalytic domain lead to asymmetry during both cell growth and division, whilst cells overexpressing Pom1p form additional growing ends. Manipulations of kinase activity indicate a negative role for Pom1p in microtubule growth at cell ends. Pom1p is present in a large protein complex and requires its non-catalytic domain to localize to the cell periphery and its kinase activity to localize to cell ends. These data establish that Pom1p kinase activity plays an important role in generating cellular symmetry and suggest that there may be related roles of homologous protein kinases ubiquitously present in all eukaryotes.
Keywords: cell polarity/Dyrk/microtubules/protein kinase/Schizosaccharomyces pombe
Introduction
Proper cell function in both unicellular and multicellular organisms depends on an appropriate spatial organization. Symmetry and asymmetry are important for morphogenesis of both single cells and whole organisms. Symmetry is required to propagate and maintain given cell structures (e.g. during the growth and division of differentiated cells), whereas asymmetry can create new and specialized cell functions (e.g. in polarized epithelial cells or during asymmetric divisions leading to cellular differentiation). Both symmetrical and asymmetrical cellular organization require positional information within the cell for the proper orientation of polarity axes. The mechanisms that generate and maintain positional information and cell polarity are not yet well understood, but seem to involve extra- or intracellular spatial cues, the cytoskeleton, and signal transduction pathways (Drubin and Nelson, 1996; Shulman and St Johnston, 1999).
The cylindrical cells of the fission yeast Schizo saccharomyces pombe show defined morphological transitions during the cell cycle, making it a simple and attractive model system to study various aspects of cellular morphogenesis (for reviews see Nurse, 1994; Bähler and Peter, 2000). Fission yeast cells grow by straight extension at their ends and divide by medial fission, creating two equal daughter cells. After cell division, both daughter cells will first grow only from the end that existed in the mother cell prior to division. Early in G2 phase, the ‘new’ end created by the preceding cell division will initiate growth, and the cells thus grow in a symmetrical way throughout the remaining interphase. The switch from unipolar to bipolar growth is known as ‘new end take off’ (NETO; Mitchison and Nurse, 1985). Cellular symmetry is also important late in the cell cycle when a cell division site is chosen at the cell centre and both daughter cells inherit an old end that has grown in the previous cycle. The sites of both polarized growth and cell division are reflected by a corresponding distribution of the actin cytoskeleton (Marks and Hyams, 1985), while microtubules are crucial for directing polarized growth towards the opposite cell ends (Mata and Nurse, 1998; Sawin and Nurse, 1998, and references cited therein). Several proteins are positioned at microtubule ends and control microtubular dynamics and polarized cell growth, including Tea1p (Mata and Nurse, 1997), Mal3p (Beinhauer et al., 1997), Tea2p (Browning et al., 2000) and Tip1p (Brunner and Nurse, 2000).
The pom1 gene has been identified in a screen for mutants with aberrations in cell growth and division. Its gene product, Pom1p, is required for the switch to bipolar growth and for the proper selection of the cell division site (Bähler and Pringle, 1998; Bähler et al., 1998a). Pom1p is a 1087-amino-acid protein with a predicted kinase catalytic domain near its C-terminus (amino acids 699–995). It is a member of an emerging family of protein kinases that are related to the MAP and Cdk kinases (Becker and Joost, 1999; Miyata and Nishida, 1999; Himpel et al., 2000). Other family members include Saccharomyces cerevisiae Yak1p (Garrett and Broach, 1989), Dictyostelium YakA (Souza et al., 1998), and the Dyrk and Minibrain kinases encoded by several genes in Drosophila, rat, mouse and human cells (Tejedor et al., 1995; Kentrup et al., 1996; Song et al., 1996; Smith et al., 1997; Becker et al., 1998; Leder et al., 1999), as well as other unpublished members present in the sequence databases. Outside their catalytic domains, these kinases show little homology except for a region immediately upstream of the catalytic domain. Several members of this protein kinase family have dual specificity for serine/threonine and tyrosine (Kentrup et al., 1996; Becker et al., 1998). Not much is known about their cellular functions, but the available data point to regulatory roles during cell growth and/or development (Becker and Joost, 1999; Lee et al., 2000). Drosophila Minibrain is involved in post-embryonic brain development, and its human counterpart maps to the ‘Down syndrome critical region’ on chromosome 21 and is implicated in the learning defects associated with trisomy 21 (Tejedor et al., 1995; Smith et al., 1997, and references cited therein).
Here we show that Pom1p has kinase activity, which is regulated during the cell cycle in correlation with the symmetry state of cells. Pom1p kinase activity is required for both symmetrical cell growth and symmetrical division, as well as for proper microtubule organization. An excess of Pom1p kinase activity at the cell ends leads to the formation of additional growing ends. Pom1p is part of a multiprotein complex, and both its non-catalytic domain and kinase activity are required for specific aspects of Pom1p localization to cell ends. We propose that the Pom1p kinase provides a signal triggering cellular symmetry, and discuss possible regulatory mechanisms that could generate cellular symmetry.
Results
Pom1p has kinase activity in vitro
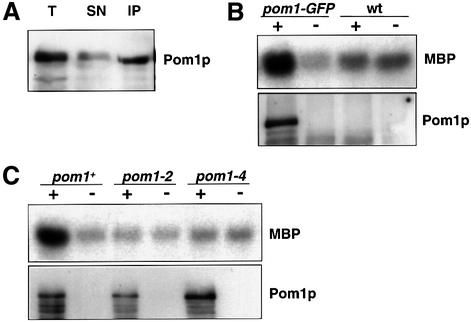
The pom1 gene has been identified in a mutant screen for abnormalities in polarized growth and cell division; it encodes a protein with a region homologous to the catalytic domain of protein kinases (see Introduction). To test whether Pom1p has kinase activity, we developed an in vitro kinase assay. Pom1p was immunoprecipitated with antibodies to green fluorescent protein (GFP) from a strain expressing GFP-tagged Pom1p at endogenous levels (Figure 1A). For some experiments, Pom1p was precipitated using either α-Pom1p antibodies or α-haemagglutinin (HA) antibodies from a strain expressing HA-tagged Pom1p (e.g. Figure 4C). The results were similar in all cases, but precipitation with antibodies against GFP gave the most reproducible protein yields. The GFP- and HA-tagged Pom1p fully rescued a pom1 deletion, showing that the tags did not significantly influence Pom1p function.
Fig. 1. In vitro protein kinase activity of Pom1p. (A) Immuno precipitation of Pom1p. Protein from strain JB115 (pom1-GFP) was extracted and precipitated using polyclonal α-GFP antibodies. The western blot shows aliquots of total extract (T), supernatant (SN) and precipitate (IP) loaded in equal quantities and probed with monoclonal α-GFP antibodies. Pom1p–GFP runs at ∼150 kDa. (B) Protein kinase assay of Pom1p. Protein extracts from strain JB115 (pom1-GFP) and the untagged 972 wild-type strain (wt) were precipitated in the presence (+) or absence (–) of α-GFP antibodies. The autoradiograph shows the Pom1p-associated phosphorylation of MBP, and western blot of the same samples probed with α-GFP antibodies to detect tagged Pom1p. (C) Absence of kinase activity in pom1 kinase mutants. Protein extracts of strains JB115 (pom1-GFP), and two mutant strains JB310 (pom1-2-GFP) and JB312 (pom1-4-GFP), which have point mutations in the ATP binding site and the activation loop, respectively, of the pom1 kinase domain, were precipitated in the presence (+) and absence (–) of α-GFP antibodies. Autoradiograph and western blot as in (B).
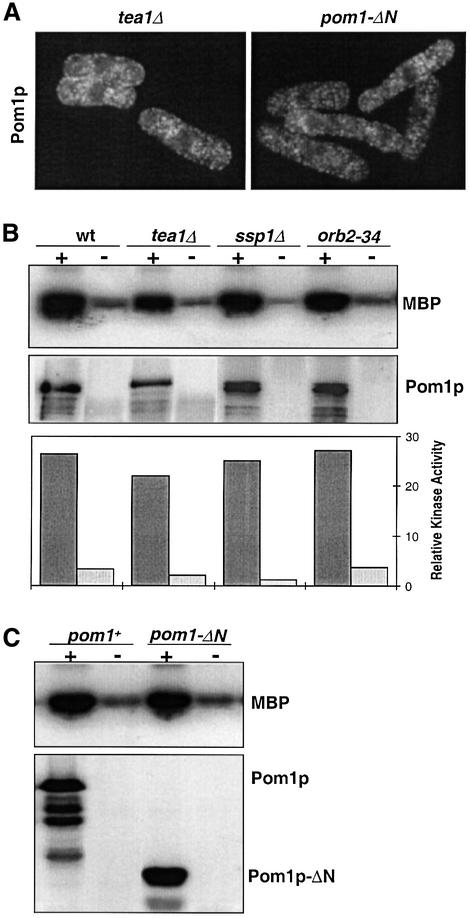
Fig. 4. Pom1p kinase localization and regulation. (A) Pom1p requires its N-terminal half and Tea1p to localize to the cell ends. Immuno fluorescence of strain JB321 (pom1-GFP tea1Δ) using α-GFP antibodies (left) and of strain JB176 (3HA-pom1-ΔN) using α-HA antibodies (right). Note that Pom1p localizes in speckles throughout the cytoplasm in both strains. The dark round regions in the cell centre correspond to the nuclei. (B) Pom1p protein kinase activity is independent of three known genes that are required for bipolar growth. Protein extracts of strains JB115 (pom1-GFP, wt), JB321 (pom1-GFP tea1Δ), JB325 (pom1-GFP ssp1Δ) and JB324 (pom1-GFP orb2-34) were precipitated in the presence (+) or absence (–) of α-GFP antibodies. Autoradiograph showing the Pom1p-associated phosphorylation of MBP, and a western blot of the same samples probed with α-GFP antibodies to detect tagged Pom1p. The graph at the bottom shows the quantitation of kinase activities with a PhosphoImager in the presence (dark grey: normalized for the amount of Pom1p) and absence (light grey) of α-GFP antibodies. (C) Pom1p protein kinase activity is independent of its N-terminal half. Protein extracts of strains JB175 (3HA-pom1+) and JB176 (3HA-pom1-ΔN) were precipitated in the presence (+) or absence (–) of α-HA antibodies. Autoradiograph and western blot as in (B).
The precipitated Pom1p had in vitro protein kinase activity against histone, casein and myelin basic protein (MBP) (Figure 1B). No significant phosphorylation was detected in the absence of Pom1p or when Pom1p was mutated within the kinase domain (Figure 1B and C). We optimized the conditions of the kinase assay such that Pom1p showed reproducible in vitro kinase activity with measurements within a linear range (see Materials and methods). Pom1p phosphorylated MBP more efficiently than either histone or casein (data not shown), and we used MBP as a substrate in all subsequent studies. This substrate specificity is similar to those of other protein kinases of the same family (Himpel et al., 2000; Kassis et al., 2000). We conclude that Pom1p can phosphorylate proteins in vitro and therefore is likely to function as a protein kinase in vivo.
Pom1p kinase activity correlates with cell symmetry
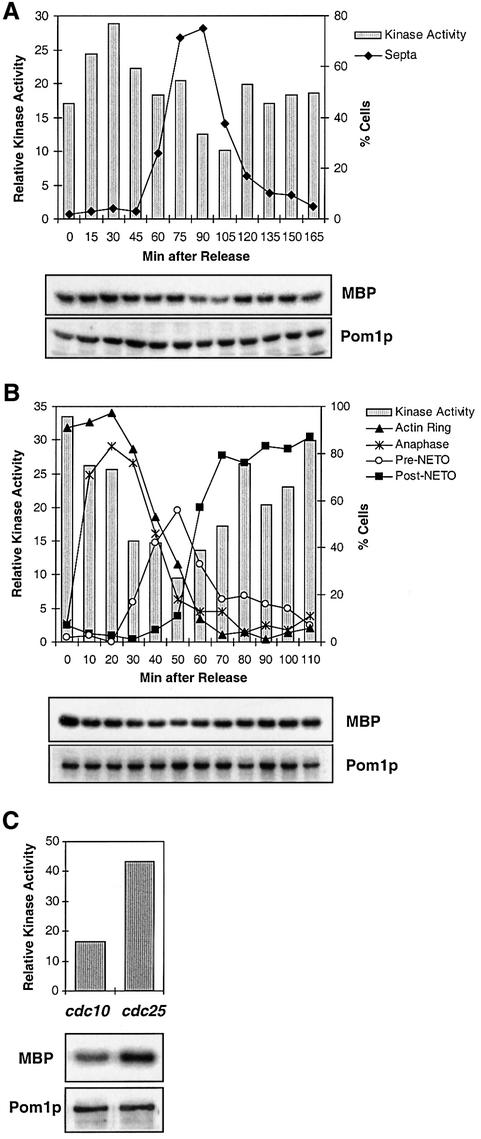
To determine whether Pom1p kinase activity is regulated during the cell cycle, cell growth was synchronized using conditional mutants. Figure 2A shows a typical experiment where cells were arrested in late G2 phase using the temperature-sensitive cdc25-22 mutant, then released to the permissive temperature, and an aliquot of synchronized cells was harvested before and every 15 min after release. For each time point, we determined the amount of Pom1p and its kinase activity as well as the percentage of cells showing a division septum. Pom1p was present throughout the cell cycle, but its associated kinase activity showed significant changes. Notably, there was a transient decrease in Pom1p kinase activity at and shortly after the peak in septation (90–105 min). This suggests that the Pom1p kinase may be less active in freshly born cells, raising the possibility that the kinase activity is down-regulated in cells growing at just one end.
Fig. 2. Cell cycle regulation of Pom1p protein kinase activity. (A) The temperature-sensitive strain JB318 (pom1-GFP cdc25-22) was grown in EMM2 medium to OD595 0.15, shifted to the restrictive temperature (36°C) for 4 h and then released to the permissive temperature (25°C). Aliquots of cells were harvested immediately before and every 15 min after the shift to 25°C. At each time point, the percentage of septated cells was determined by Calcofluor staining. Protein extracts from each time point were precipitated with α-GFP antibodies and analysed for Pom1p kinase activity (MBP) and the presence of Pom1p. The kinase activities were quantified with a PhosphoImager and normalized for the amount of Pom1p present. (B) The cold-sensitive strain JB319 (pom1-GFP nda3-KM311) was grown in YE medium to OD595 0.3, shifted to the restrictive temperature (20°C) for 6 h and then released to the permissive temperature (32°C). Aliquots of cells were harvested immediately before and every 10 min after the shift to 32°C. For each time point, cell cycle stages were determined by DAPI staining to check for anaphase (presence of two nuclei) and by phalloidin staining to check for cytokinesis, pre- and post-NETO cells (presence of F-actin ring, unipolar and bipolar actin distribution, respectively). Pom1p kinase activities (MBP) and the presence of Pom1p were analysed at each time point as in (A). (C) The temperature-sensitive strains JB309 (pom1-GFP cdc10–129) and JB318 (pom1-GFP cdc25-22) were grown in EMM medium to OD595 0.2 and shifted to the restrictive temperature (36°C) for 3.5 h. Pom1p protein kinase activities in the arrested cells were determined as in (A). cdc10 and cdc25 cells arrest before and after the switch to bipolar growth, respectively (Mitchison and Nurse, 1985).
cdc25 cells are highly elongated after the arrest, and initiate both S-phase and the switch to bipolar growth almost immediately after cell division (data not shown). To test the idea that Pom1p kinase activity is lower in unipolar cells, we synchronized the cells by mitotic arrest using the cold-sensitive tubulin mutant nda3-KM311 (Figure 2B). These cells become far less elongated at the restrictive temperature, allowing a more extended unipolar growth phase than in the cdc25-22 experiment. Before and every 10 min after release to the permissive temperature, an aliquot of the synchronized cells was harvested. For each time point, we determined the amount of Pom1p and its kinase activity as well as cytological landmarks of various cell cycle stages. The Pom1p kinase activity was high at the time of mitotic arrest and remained high until ∼20 min after release (when the majority of cells underwent anaphase). There was a fall in kinase activity at about the time the first new cells created by cell division were starting to grow in a unipolar manner from their old ends (30 min). Pom1p kinase activity was lowest when the number of unipolar (pre-NETO) cells peaked (50 min) and then started to increase again (from 60 min), coincidently with cells switching to bipolar growth (post-NETO).
Compared with the cdc25 experiment (Figure 2A), the fall in kinase activity after cell division was more pronounced and extended (Figure 2B), in accordance with the longer unipolar growth phase in the latter experiment. It is difficult to be certain of the kinase activity pattern relative to cell cycle stages in these experiments, but we performed both the cdc25 and nda3 time course experiments three times independently, and in each case the kinase activity was found to be lowest after cell division and to increase again at about the time of the switch to bipolar growth. Similarly, a cdc10 arrest-release experiment gave results consistent with the other synchronizations: kinase activity was low during arrest and the first time points after release, but increased after completion of S-phase and coincident with the switch to bipolar growth (data not shown). These experiments suggest that Pom1p kinase activity is higher when cells are growing in a bipolar manner or undergoing symmetrical cell division, whereas Pom1p kinase activity is reduced when cells are growing asymmetrically. This view is further supported by the observation that Pom1p kinase activity was significantly lower in cdc10 cells arrested in G1 phase before the switch to bipolar growth compared with cdc25 cells that were arrested in G2 phase during bipolar growth (Figure 2C). We conclude that Pom1p kinase activity is regulated during the cell cycle and that the symmetry state of the cells correlates with Pom1p kinase activity. The activity is up-regulated in cells that are organized symmetrically, i.e. during bipolar growth and cell division, and down-regulated in cells growing in a unipolar manner.
Role of Pom1p kinase activity in vivo
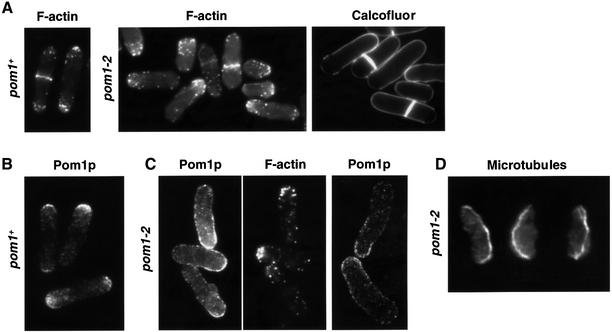
To investigate whether increased Pom1p kinase activity is necessary to generate cell symmetry or is a consequence of cell symmetry, we examined the effects of point mutations in conserved amino acids of the Pom1p catalytic domain. Three strains were created by in vitro mutagenesis: pom1-2 (Lys728Arg), pom1-3 (Tyr857/9Asp) and pom1-4 (Tyr857/9Phe). The pom1-2 mutation is in the ATP-binding site of the catalytic domain known to be critical for kinase function. Corresponding mutations in other kinases lead to complete defects in their catalytic activity (Hanks et al., 1988; Garrett et al., 1991). In pom1-3 and pom1-4, both tyrosines in the ‘activation loop’ between subdomains VII and VIII were exchanged for other residues. In the homologous Dyrk kinase, the corresponding tyrosines need to be phosphorylated for the kinase to be active (Kentrup et al., 1996). With the exchange of tyrosine for aspartic acid, we hoped to mimic the phosphorylated state and create a hyperactivated mutant, whereas with the exchange for phenylalanine, we expected to create a ‘unactivatable’ and thus kinase-dead mutant. However, all three mutants completely lacked protein kinase activity in our in vitro assay system (Figure 1C and data not shown). We then investigated the phenotype of these kinase-dead mutants. All mutants showed phenotypes similar to the pom1 deletion mutant (Bähler and Pringle, 1998), i.e. a defect in the switch to bipolar growth and asymmetrically placed septa (Figure 3A and data not shown). Less than 10% of the mutant cells showed F-actin distributed symmetrically at both ends (and thus bipolar growth) compared with >50% for wild-type cells. We conclude that the kinase activity is crucial for Pom1p function in vivo, and that it is required to generate symmetry during both cell growth and cell division.
Fig. 3. Cellular functions of Pom1p protein kinase activity. (A) Defects in both symmetrical cell growth and cell division in a strain mutated in the pom1 kinase domain. Strain JB179 (pom1-2) was stained for F-actin with phalloidin and for cell wall growth with Calcofluor. Phalloidin-stained 972 wild-type cells (pom1+) are shown for comparison. Note that F-actin is concentrated at one end only and actin rings are placed off centre in the mutant cells. Consequently, cell wall growth is limited to one end and division septa are formed asymmetrically. (B) Localization of Pom1p to the cell ends. Immunofluorescence of strain JB115 (pom1+-GFP) using α-GFP antibodies. Note that Pom1p is concentrated at cell ends. (C) Pom1p fails to localize exclusively to cell ends in a strain mutated in the kinase domain. Immunofluorescence of strain JB310 (pom1-2-GFP) using α-GFP and α-actin antibodies. Note that the mutated Pom1p localizes in a wide area in one half of the cell opposite to the end where F-actin is concentrated. The confocal section on the right shows that Pom1p is located at the cell periphery without being restricted to the very cell end. (D) Microtubules bend around the cell end in the absence of Pom1p kinase activity. Immunofluorescence of strain JB179 (pom1-2) using α-tubulin antibody. Note that microtubules are curled around the broader new cell ends (bottom), extending to greater length than they would in wild-type cells.
Pom1p is localized to cell ends (Figure 3B; Bähler and Pringle, 1998). To determine whether pom1 kinase activity is required for localization, we visualized mutated Pom1p by immunofluorescence (Figure 3C). Mutant Pom1p was still found at cell ends, but additionally occupied a larger part of the cell periphery adjacent to the ends, gradually becoming weaker the further the distance from the end. The bulk of Pom1p was positioned at the periphery of the cell-half opposite the growing end, which contains F-actin (Figure 3C). This is similar to wild-type cells, which grow at only one end and have Pom1p concentrated at the non-growing end (Bähler and Pringle, 1998). Thus, although kinase-dead Pom1p can still localize to the non-growing cell end, its localization is not restricted to the very end, and it forms a gradient towards the cell middle. We conclude that the kinase activity of Pom1p is required to properly localize and/or maintain Pom1p itself at cell ends.
Pom1p requires microtubules to localize to cell ends (Bähler and Pringle, 1998). Because of the aberrant Pom1p localization in the absence of kinase activity, we looked at microtubule organization in pom1 kinase mutants (Figure 3D). A substantial fraction of cells (∼10%) showed long microtubules curling around cell ends; in most cases, the microtubules were curled around the non-growing end, which is broader than the growing end (lower end in Figure 3D). Thus, microtubule growth seems to be affected in the absence of Pom1p. Although this defect in microtubule organization is subtle, it might explain why Pom1p is not precisely localized to cell ends in the absence of kinase activity.
Pom1p kinase location and regulation
It is possible that an interaction with another protein localized at the cell end leads to activation of the Pom1p kinase during NETO and growth at the new end. We therefore examined whether Pom1p localization to the cell ends is required for the kinase to become active. Because Pom1p does not localize to the cell ends in the absence of Tea1p (Bähler and Pringle, 1998), we measured Pom1p kinase activity in a tea1 deletion mutant. In this background, Pom1p localizes in irregular speckles throughout the cytoplasm (Figure 4A). However, the in vitro kinase activity of Pom1p was not significantly affected by the absence of Tea1p, but was similar to the activity in a wild-type background (Figure 4B). We conclude that Pom1p does not need to be localized at the cell end for the kinase to be active. Two other protein kinases are known to be required for the switch to bipolar growth: Ssp1p (Matsusaka et al., 1995; Rupes et al., 1999) and Orb2p/Pak1p/Shk1p (Verde et al., 1995, 1998). To see whether these kinases regulate Pom1p, we measured Pom1p kinase activity in ssp1 and orb2 mutant strains. However, Pom1p kinase activity was very similar to a wild-type strain in both cases (Figure 4B). In conclusion, Pom1p kinase activity is independent of three other proteins (Tea1p, Ssp1 and Orb2p) that are required for bipolar growth.
Pom1p is a relatively large protein (1087 amino acids), yet a single point mutation in the kinase domain leads to the same defects as a complete gene deletion (see above). To gain insight into the role of the large N-terminal part upstream of the catalytic domain, we deleted the first 1770 bp of pom1 (corresponding to >50% of the gene). This partial deletion mutant showed a very similar phenotype to the complete deletion and to the kinase-dead mutants, i.e. asymmetric septa and unipolar cell growth (data not shown). The in vitro kinase activity of the N-terminally truncated Pom1p was similar to that of the full-length Pom1p (Figure 4C). However, in these mutants, Pom1p completely failed to localize to the cell ends but was localized in dots throughout the cytoplasm, as in the tea1 deletion background (Figure 4A). Thus, while the N-terminal domain is not required for kinase activity, it is essential for proper localization of Pom1p.
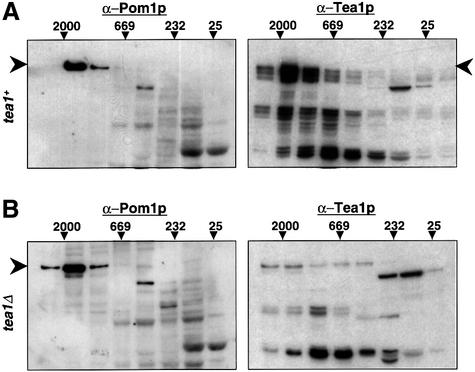
The results above suggest that Pom1p is associated with other proteins. Indeed, Pom1p is detected in a high molecular weight complex of >1000 kDa in gel filtration experiments (Figure 5A). The size of this complex does not change significantly during the cell cycle (data not shown). Because the Pom1p complex is similar in size to the complex containing Tea1p (Figure 5A; Mata and Nurse, 1997), we performed gel filtration experiments on Pom1p in a tea1 deletion background. However, the size of the Pom1p-containing complex remained unchanged in the absence of Tea1p (Figure 5B). Since Pom1p is not localized at cell ends in this background (Figure 4A), we conclude that Pom1p is found in a large complex independent of its location at the cell ends and independent of Tea1p.
Fig. 5. Pom1p is present in a high molecular mass complex similar to but independent of Tea1p. (A) Protein extracts of strain JB115 (pom1-GFP) were fractionated by gel filtration. The fractions were analysed on western blots probed with α-GFP (to detect Pom1p) or α-Tea1p antibodies. The migration of molecular mass markers is shown on top (values in kilodaltons). Arrowheads indicate the positions of full-length Pom1p and Tea1p. Note that the Pom1p and Tea1p complexes migrate at a similar molecular mass. (B) Protein extracts of strain JB321 (pom1-GFP tea1Δ) were fractionated by gel filtration and analysed as in (A). The α-Tea1p antibody cross-reacts with various other proteins [compare to (A)].
Overexpression of Pom1p kinase
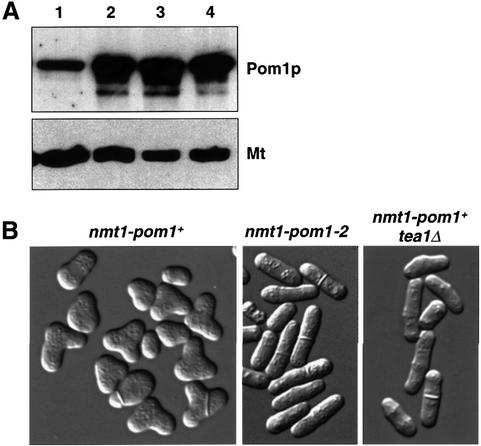
To examine the effect of excess Pom1p kinase, we put pom1 under the control of the strong thiamine-regulated nmt1 promoter (see Materials and methods; Maundrell, 1990). By 16 h after thiamine removal, ∼4 h after induction of the promoter, cells had started to grow by branching from their sides. By 19 h, Pom1p was present in clearly higher levels than in wild-type cells (Figure 6A), and ∼80% of the cells were branched (‘T-shaped’; Figure 6B). At later times, cells started to swell, and 25 h after induction most cells were large and round with actin distributed in a depolarized way all over the cell surface (data not shown). These changes in cell shape were dependent on Pom1p kinase activity: cells overexpressing a kinase-dead mutant maintained a normal cylindrical shape (Figure 6A and B).
Fig. 6. Overexpression of Pom1p protein kinase leads to cell branching. (A) Western blot probed with α-Pom1p antibody. Protein extracts of the following strains were loaded: (1) 972 (wild type); (2) JB151 (nmt1-pom1+); (3) JB314 (nmt1-pom1-2); (4) JB322 (nmt1-pom1+ tea1Δ). The same blot was probed with an α-tubulin antibody as a loading control (Mt). Strains with pom1 under the control of the nmt1 promoter were grown in the absence of thiamine for 19 h as described in (B). (B) Cells of strains JB151 (nmt1-pom1+), JB314 (nmt1-pom1-2) and JB322 (nmt1-pom1+ tea1Δ) were grown in EMM2 + thiamine, shifted to EMM2 without thiamine to induce the nmt1 promoter for 19 h at 32°C. Micrographs were taken using differential interference contrast microscopy. Note that cell branching depends both on Pom1p kinase activity and on Tea1p.
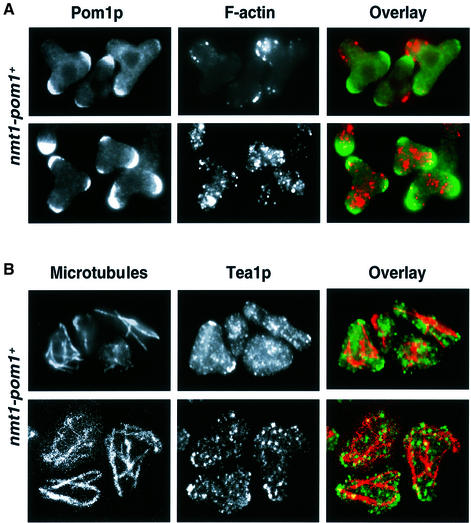
We also examined the location of Pom1p when it was overexpressed: 16 h after inducing the promoter, Pom1p was highly concentrated at both original cell ends, whereas most actin was localized in the branched cell end (Figure 7A, top row). This high amount of Pom1p at original cell ends appeared to be the reason for cell branching: cells overexpressing Pom1p in a tea1Δ background were of normal shape (Figure 6A and B), presumably because Pom1p fails to localize to the ends in the absence of Tea1p. Nineteen hours after inducing the promoter, Pom1p was concentrated at each of the three cell ends, and F-actin had lost its polarized localization and became distributed all over the cells (Figure 7A, bottom row). We conclude that an excess of Pom1p kinase at cell ends prevents these ends from growing, leading to growth from the cell middle and eventually to completely depolarized cell growth.
Fig. 7. Cytoskeletal aberrations in cells overexpressing Pom1p. (A) Immunofluorescence of strain JB151 (nmt1-pom1+) using α-Pom1p and α-actin antibodies. Note that at 16 h after induction of the nmt1 promoter (top row), Pom1p is strongly localized at the two normal cell ends, whereas F-actin is mainly concentrated in the branching cell end. At 19 h after induction of the nmt1 promoter (bottom row), Pom1p is localized at all three cell ends, and the F-actin localization has become depolarized. (B) Immunofluorescence of strain JB151 using α-tubulin and α-Tea1p antibodies. The nmt1 promoter was induced for 19 h. Immunofluorescence micrographs (top row) and confocal micrographs (bottom row) are shown. The microtubules are shorter and aberrantly organized, and Tea1p fails to become highly concentrated at the cell ends when Pom1p is overexpressed.
To determine how excess Pom1p kinase might prevent cell ends from growing, we examined microtubules and Tea1p localization in cells overexpressing Pom1p (Figure 7B). Microtubules appeared shorter than in wild-type cells and seemed to run in random directions within the cells. Tea1p is normally concentrated at cell ends by microtubular transport (Mata and Nurse, 1997). Notably, Tea1p appeared to have problems localizing correctly when Pom1p was overexpressed. As Pom1p levels started to increase 16 h after inducing the overexpression, most cells still had some Tea1p located at their normal ends (not shown), but 3 h later almost all Tea1p was randomly distributed in the cytoplasm (Figure 7B). The defects in microtubule organization and Tea1p localization provide an explanation for the cell branching caused by Pom1p overexpression. These results together with those shown in Figure 3D indicate that the Pom1p kinase plays some role in microtubule regulation, possibly providing a negative signal for microtubule growth at cell ends.
Discussion
This paper addresses the cellular functions and regulation of Pom1p in fission yeast. We show that Pom1p has protein kinase activity, and that this activity is regulated during the cell cycle in correlation with the state of cellular symmetry, i.e. the activity is high in cells growing and dividing symmetrically, and lower in newly born cells that grow asymmetrically. This correlation is evident in cells synchronized in the cell cycle by different methods and in cells arrested before or after the switch to bipolar growth. Point mutants in the catalytic domain lacking Pom1p kinase activity show defects in symmetrical cell growth and in symmetrical cell division. Thus, Pom1p kinase activity is essential for cellular symmetry during both growth and division, and may provide a signal that triggers symmetry. We propose that up-regulation of Pom1p kinase activity after S-phase is instrumental for the switch to bipolar growth. The high activity of Pom1p kinase throughout the rest of interphase and mitosis might then be required to maintain bipolar growth and to position the division site in the cell middle.
Normally, the switch to bipolar growth depends both on a minimal cell size and on completion of S-phase (Mitchison and Nurse, 1985). It is, therefore, likely that the cell cycle machinery directly or indirectly provides the signal to activate Pom1p. The possibility that a protein at the cell end is required for activation can be eliminated because Pom1p has high kinase activity even when localized in the cytoplasm, as shown in the absence of either its non-catalytic domain or Tea1p. Pom1p is present in a high molecular weight protein complex of similar size to that seen with Tea1p. However, the Pom1p complex remains unchanged in a tea1 deletion mutant, suggesting that Pom1p is present in the protein complex independent of Tea1p and of its own localization to cell ends. Pom1p does not co-immunoprecipitate with itself in a diploid strain containing two differently tagged versions of the protein, suggesting that other proteins are present in the complex (our unpublished data). This protein complex might contain regulators of Pom1p. The two protein kinases Ssp1p and Orb2p/Pak1p/Shk1p are also required for the switch to bipolar growth (Matsusaka et al., 1995; Verde et al., 1995, 1998; Rupes et al., 1999). The finding that Pom1p kinase activity was similar to wild type, in both ssp1 and orb2 mutant strains, suggests that Pom1p acts upstream of these kinases. Other members of the Dyrk kinase family autophosphorylate the tyrosine residues in the ‘activation loop’, which is required for kinase activity (Kentrup et al., 1996; Kassis et al., 2000), and we also observed phosphorylation of Pom1p in our kinase assays (unpublished data). Pom1p might therefore be involved in its own activation through a positive feedback loop, leading to a rapid and irreversible up-regulation of kinase activity once initiated.
Because microtubules are critical for establishing and directing cellular polarity in fission yeast (Sawin and Nurse, 1998; Bähler and Peter, 2000, and references cited therein), it is possible that Pom1p activates bipolar growth through its effect on microtubules. Cells lacking Pom1p kinase activity often have longer curved microtubules, while cells overexpressing Pom1p have short and aberrant microtubules. These effects on microtubule behaviour are similar to those reported for Tea1p (Mata and Nurse, 1997) and suggest that the Pom1p kinase is involved in a negative signal for microtubule growth at cell ends. Pom1p is concentrated at the new cell end before the switch to bipolar growth (Bähler and Pringle, 1998), and activation of its kinase activity might generate a microtubule configuration at the new end that triggers growth.
Altered microtubules could also explain the branching of cells overexpressing Pom1p. An excess of Pom1p kinase at cell ends eventually prevents Tea1p from accumulating at these ends, probably due to the shortened and disorganized microtubules. These alterations in the Tea1p–microtubule organization could then cause repolarization of F-actin, leading to the formation of an aberrant growth site in the middle region of the cell. After prolonged overexpression, Pom1p begins to accumulate also at this third abnormally placed cell end, and F-actin becomes depolarized, resulting eventually in rounded cells. Pom1p kinase activity and Tea1p are both essential to generate this phenotype. How do Tea1p and Pom1p relate to each other? Pom1p requires Tea1p for proper localization, whereas Tea1p localizes normally in the absence of Pom1p, and tea1 pom1 double mutants show no stronger defect than either single mutant (Bähler and Pringle, 1998). These findings suggest that Pom1p acts downstream of Tea1p, and in fact many phenotypes of tea1 mutants (unipolar growth, microtubule defects, cell branching) could be explained by the failure to localize Pom1p properly to cell ends. We propose that Pom1p acts in the same pathway as the Tea1p–microtubule system and is an important effecter of this system at cell ends.
What substrates of Pom1p are responsible for its effect on microtubules? Recently, a Clip170-like protein, Tip1p, has been identified that is located at the ends of growing microtubules and prevents microtubule shrinkage before they reach the cell ends (Brunner and Nurse, 2000). Clip170 proteins in multicellular organisms need to be phosphorylated to fall off the ends of growing microtubules (Rickard and Kreis, 1996). An attractive hypothesis would be that Pom1p phosphorylates Tip1p at cell ends and thus triggers microtubule shrinkage. It is also possible that Pom1p acts indirectly by phosphorylating substrates at the cell ends, which then trigger bipolar growth either by reorganizing microtubules and/or by a microtubule-independent mechanism. Both symmetrical cell growth and symmetrical division depend on the activity of Pom1p kinase, raising the possibility that both processes share a common mechanism. One defect is not just an indirect effect of the other, because mutants exist with specific defects in symmetrical growth but not division (e.g. tea1 and orb2: Verde et al., 1995) or vice versa (e.g. mid1 and plo1: Chang et al., 1996; Sohrmann et al., 1996; Bähler et al., 1998a). It is not clear at present whether symmetrical growth and division share other factors or common processes.
Pom1p itself requires microtubules to localize to cell ends (Bähler and Pringle, 1998). It might, therefore, be part of a positive feedback mechanism whereby microtubules guide Pom1p to cell ends; Pom1p could then help the microtubules to recognize and reinforce that end, as has been suggested for Tea1p (Mata and Nurse, 1997). Remarkably, the kinase activity of Pom1p is essential for its own localization to cell ends. The long bent microtubules extending beyond cell ends in pom1 kinase mutants could explain why Pom1p is not localized exclusively to the ends in these mutants, but is found along the cell periphery close to the end. Alternatively, kinase activity might be required to anchor Pom1p at the cell ends. Although the localization of Pom1p differs between wild-type and kinase mutant backgrounds, it is found at the cell periphery in both cases, suggesting that Pom1p associates with the cell membrane. Consistent with this is the finding that a detergent in the lysis buffer solubilizes Pom1p (Materials and methods). Furthermore, Pom1p co-localizes with the cell membrane in immunogold electron micrographs (S.Gschmeissner and J.Bähler, unpublished results), and it seems to have a higher affinity for the cell periphery than for microtubules because it remains at cell ends in the absence of normal microtubule organization (Figure 7). Pom1p does not have a trans-membrane domain, but might be anchored to a membrane protein similar to other protein kinases such as PKC (Mochly-Rosen, 1995). Localization of Pom1p to the cell periphery requires its non-catalytic domain; this domain might bind a membrane-associated protein, and/or it might link Pom1p to microtubules, e.g. via Tea1p. Consistent with the latter idea, Pom1p shows similar dotted cytoplasmic staining both in the absence of Tea1p and when the non-catalytic domain is deleted.
Pom1p is a member of the conserved Dyrk family of protein kinases, which play ill-defined roles during cell growth and development (see Introduction). There is also at least one other fission yeast member of this family, which has been identified by the genome project (SPAC16C9.07; Sanger Centre). Outside the kinase domain, Pom1p has additional sequence similarity with some family members in multicellular eukaryotes (Dyrk and Minibrain) in a region immediately upstream of the catalytic domain. Budding yeast has just one member of this family (Yak1p; Becker and Joost, 1999), which shows no homology outside the catalytic domain. It is interesting to speculate that budding yeast, which grows with a single axis of polarity throughout the cell cycle and divides asymmetrically by budding, might not need a functional homologue of Pom1p. In multicellular eukaryotes, some of the Dyrk kinases might play roles similar to Pom1p in polarized growth and symmetrical division. Intriguingly, Minibrain functions in post-embryonic neurogenesis and is implicated in the mental retardation associated with trisomy 21 (see Introduction). Clearly, cell polarity is crucial for nerve cell function, and an extra copy of this kinase could create problems similar to the aberrations seen when Pom1p is overexpressed.
Materials and methods
Schizosaccharomyces pombe strains and methods
A list of strains used is given in Table I. pom1 was epitope tagged or put under the control of the nmt1 promoter using a direct PCR-based approach and the kanMX6 dominant marker (Bähler et al., 1998b). To minimize crosses, the PCR tagging was performed in parallel directly into the various strain backgrounds. Standard growth media and fission yeast methods were used (http://www.bio.uva.nl/pombe/handbook/; Moreno et al., 1991).
Table I. Fission yeast strains used in this study.
| Strain | Genotype | Source/reference |
|---|---|---|
| 972 | wild-type h– | Leupold (1970) |
| JB115 | pom1-GFPS65T h– | Bähler and Pringle (1998) |
| JB107 | pom1-Δ1::ura4+ ura4-D18 leu1-32 h– | this study |
| JB179 | pom1-2 (K728R) leu1-32 ura4-D18 h– | this study |
| JB180 | pom1-3 (Y857/859D) leu1-32 ura4-D18 h– | this study |
| JB181 | pom1-4 (Y857/859F) leu1-32 ura4-D18 h– | this study |
| JB310 | pom1-2-GFPS65T leu1-32 ura4-D18 h– | this study |
| JB312 | pom1-4-GFPS65T leu1-32 ura4-D18 h– | this study |
| JB319 | pom1-GFPS65T nda3-KM311 h– | this study |
| JB309 | pom1-GFPS65T cdc10–129 h– | this study |
| JB318 | pom1-GFPS65T cdc25-22 h– | this study |
| JB321 | pom1-GFPS65T tea1Δ h– | this study |
| JB324 | pom1-GFPS65T orb2-34 h– | this study |
| JB325 | pom1-GFPS65T ssp1Δ h– | this study |
| JB175 | 41nmt1-3HA-pom1 h– | this study |
| JB176 | 41nmt1-3HA-pom1-ΔN h– | this study |
| JB151 | 3nmt1-pom1 h– | this study |
| JB314 | 3nmt1-pom1-2 leu1-32 ura4-D18 h– | this study |
| JB322 | 3nmt1-pom1 tea1Δ h– | this study |
Site-directed mutagenesis of the pom1 catalytic domain
Three mutant strains with defined mutations in the kinase catalytic domain of pom1 were created by in vitro mutagenesis using the QuikChange kit (Stratagene) and the plasmid pJB1 (the full-length pom1 gene together with 570 and 430 bp of up- and downstream sequences, respectively, cloned into the PstI and KpnI sites of pUR19). The point mutations were introduced according to the manufacturer’s instructions using the following primers (mutations are underlined): 5′-CTGGAAAACTTGTTGCATTGAGAATTATCCGTAATAA-3′ for pom1-2 (K728R), 5′-GAAGGTGAATGCGTTGATACAGACATTCAATCTCGGTTTTATCG-3′ for pom1-3 (Y857/859D) and 5′-GAAGGTGAATGCGTTTTTACATTCATTCAATCTCGGTTTTATCG-3′ for pom1-4 (Y857/859F) together with the corresponding reverse primers. The mutated plasmids were amplified in bacteria using a Miniprep kit (Qiagen), and the mutations were confirmed by sequencing. The plasmids were digested with PstI and KpnI, phenol extracted, precipitated, transformed into strain JB107 (pom1Δ::ura4+ ura4-D18 leu1-32), and plated onto EMM2. After 3 days at 32°C, cells were replica plated onto YEA + 5-fluoro-orotic acid (5-FOA) to select for replacement of ura4+ by the mutated pom1 genes. Growing colonies were re-isolated on YEA + 5-FOA and checked by colony PCR for the presence of pom1 sequence. Successful introductions of the point mutations were confirmed by direct sequencing of the PCR products after Qiagen purification.
Protein preparation, gel filtration and western blotting
Native protein extracts were prepared by breaking cells with glass beads as described (http://www.bio.uva.nl/pombe/handbook/), except that the extraction buffer was optimized for the study of Pom1p [POM buffer: 25 mM HEPES 7.4, 1% Triton X-100, 10% glycerol, 50 mM potassium acetate, 50 mM NaF, 60 mM β-glycerolphosphate, 2 mM EDTA, 1 mM dithiothreitol, 0.1 mM sodium vanadate, 15 mM p-nitrophenylphosphate, 40 µg/ml aprotinin, 20 µg/ml leupeptin, 1 µg/ml pepstatin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM benzamidine]. For gel filtration experiments, extracts were prepared as above, centrifuged at 100 000 g for 30 min, and 1.5 mg of protein were loaded on a Superose 6 column using the SMART system (Pharmacia). For western blotting, extracts were separated on 6–12% SDS–PAGE gels and transferred to Immobilon-P membranes (Millipore). Antibody dilutions were 1:200 for monoclonal α-GFP (GFP 3E1; a gift of Tim Hunt) and polyclonal α-Pom1p (Bähler and Pringle, 1998), and 1:2000 for monoclonal α-HA (12CA5; Imperial Cancer Research Fund) and polyclonal α-Tea1p (Mata and Nurse, 1997). Proteins were detected using ECL reagents (Amersham).
Immunoprecipitations and in vitro kinase assays
For immunoprecipitations, 1.5 × 108 cells were harvested by filtration, washed once in STOP buffer (150 mM NaCl, 50 mM NaF, 10 mM EDTA, 1 mM NaN3) and once in POM buffer (see above), and the cell pellets were frozen in dry ice. Care was taken that cells grown in EMM2 or YE medium had no densities higher than OD595 0.5 or 0.8, respectively, at the time of harvest. Cell lysates were prepared as described above and centrifuged for 15 min at 16 000 g in a cooled microfuge. The protein extracts (in 200 µl of POM buffer) of GFP-tagged strains were incubated for 1.5 h on ice with 2 µl of polyclonal α-GFP (a gift of Ken Sawin). Protein A–Sepharose beads were washed three times in POB buffer (POM buffer + 50 mg/ml bovine serum albumin, without protease and phosphatase inhibitors) and once in POM buffer. The beads were then added to the extracts in 100 µl of POM buffer and incubated for 45 min on a rotator at 4°C. Protein extracts of HA-tagged strains were incubated with α-HA–Sepharose (BABCO) for 2 h on a rotator at 4°C. After these incubations, the Sepharose beads were washed twice with 1 ml of POM buffer and once with 1 ml of AB buffer (25 mM MOPS pH 7.0, 60 mM β-glycerolphosphate, 7 mM MgCl2, 7 mM MnCl, 0.1 mM sodium vanadate, 40 µg/ml aprotinin, 20 µg/ml leupeptin, 1 mM PMSF, 2 mM benzamidine). The beads were resuspended in 15 µl of AB buffer plus 1 mg/ml MBP (Sigma), 10 µM ATP, 3 µCi of [µ-32P]ATP. The reactions were incubated at 30°C for 20 min and stopped by adding 8 µl of 5× Laemmli buffer and incubating at 100°C for 5 min. Proteins were separated on 12% SDS–PAGE gels. Autoradiography was performed after transferring the proteins to Immobilon-P membranes. The amounts of Pom1p in the reaction mixtures were determined (using NIH image) after probing the upper part of the filter with monoclonal α-GFP antibody. Phosphorylated MBP was quantified using a PhosphoImager and normalized for the amount of Pom1p present in the assay. Control experiments were performed in the same way, but in the absence of antibodies.
Cytology
Cell wall staining by Calcofluor and DNA staining by DAPI were performed using routine methods (http://www.bio.uva.nl/pombe/handbook/). For staining of F-actin by rhodamine–phalloidin, 1 ml of cells was fixed with 333 µl of 16% EM-grade formaldehyde for 30 min at room temperature and then kept on ice until further processing as described (Sawin and Nurse, 1998). Immunofluorescence experiments using α-HA and α-Pom1p antibodies were performed as described (Bähler and Pringle, 1998), except that Alexa488 and Alexa594 secondary antibodies were used at 1:1000 (Molecular Probes). Pom1p–GFP was detected using polyclonal α-GFP antibodies at 1:200 (a gift of Ken Sawin) and double staining for F-actin was carried out using monoclonal α-actin antibodies (N350; Amersham) at 1:150 using the fixation/post-fixation protocol described before (Bähler and Pringle, 1998). For microtubule and Tea1p detection, the Tat1 monoclonal antibody (Woods et al., 1989; a gift of K.Gull) and an antibody recognizing the C-terminal half of Tea1p were used as described (Mata and Nurse, 1997). Microscopy and photography (immunofluorescence, differential interference contrast, and confocal) were performed as described by Sawin and Nurse (1998).
Acknowledgments
Acknowledgements
We thank Heidi Browning, Jacky Hayles and Takashi Toda for comments on the manuscript; Keith Gull, Tim Hunt and Ken Sawin for antibodies; and all members of the cell cycle laboratory, particularly Manolo Arellano, Damian Brunner, Jacky Hayles and Zoi Lygerou, for advice and valuable discussions. J.B. was supported by fellowships from the Imperial Cancer Research Fund and the Novartis-Stiftung.
References
- Bähler J. and Peter,M. (2000) Cell polarity in yeast. In Drubin,D.G. (ed.), Cell Polarity. Oxford University Press, Oxford, UK, pp. 21–77.
- Bähler J. and Pringle,J.R. (1998) Pom1p, a fission yeast protein kinase that provides positional information for both polarized growth and cytokinesis. Genes Dev., 12, 1356–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J., Steever,A.B., Wheatley,S., Wang,Y.-L., Pringle,J.R., Gould,K.L. and McCollum,D. (1998a) Role of Polo kinase and Mid1p in determining the site of cell division in fission yeast. J. Cell Biol., 143, 1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J., Wu,J.-Q., Longtine,M.S., Shah,N.G., McKenzie,A.M.,III, Steever,A.B., Wach,A., Philippsen,P. and Pringle,J.R. (1998b) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast, 14, 943–951. [DOI] [PubMed] [Google Scholar]
- Becker W. and Joost,H.G. (1999) Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. Prog. Nucleic Acid Res. Mol. Biol., 62, 1–17. [DOI] [PubMed] [Google Scholar]
- Becker W., Weber,Y., Wetzel,K., Eirmbter,K., Tejedor,F.J. and Joost, H.-G. (1998) Sequence characteristics, subcellular localization, and substrate specificity of DYRK-related kinases, a novel family of dual specificity protein kinases. J. Biol. Chem., 273, 25893–25902. [DOI] [PubMed] [Google Scholar]
- Beinhauer J.D., Hagen,I.M., Hegemann,J.H. and Fleig,U. (1997) Mal3, the fission yeast homologue of the human APC-interacting protein EB-1 is required for microtubule integrity and the maintenance of cell form. J. Cell Biol., 139, 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning H., Hayles,J., Mata,J., Aveline,L., Nurse,P. and McIntosh,J.R. (2000) Tea2p is a kinesin-like protein required to generate polarized growth in fission yeast. J. Cell Biol., 151, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D. and Nurse,P. (2000) CLIP170-like tip1p spatially organizes microtubular dynamics in fission yeast. Cell, 102, 695–704. [DOI] [PubMed] [Google Scholar]
- Chang F., Woollard,A. and Nurse,P. (1996) Isolation and character ization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J. Cell Sci., 109, 131–142. [DOI] [PubMed] [Google Scholar]
- Drubin D.G. and Nelson,W.J. (1996) Origins of cell polarity. Cell, 84, 335–344. [DOI] [PubMed] [Google Scholar]
- Garrett S. and Broach,J. (1989) Loss of Ras activity in Saccharomyces cerevisiae is suppressed by disruptions of a new kinase gene, YAK1, whose product may act downstream of the cAMP-dependent protein kinase. Genes Dev., 3, 1336–1348. [DOI] [PubMed] [Google Scholar]
- Garrett S., Menold,M.M. and Broach,J.R. (1991) The Saccharomyces cerevisiae YAK1 gene encodes a protein kinase that is induced by arrest early in the cell cycle. Mol. Cell. Biol., 11, 4045–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S.K., Quinn,A.M. and Hunter,T. (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science, 241, 42–52. [DOI] [PubMed] [Google Scholar]
- Himpel S., Tegge,W., Frank,R., Leder,S., Joost,H.-G. and Becker,W. (2000) Specificity determinants of substrate recognition by the protein kinase DYRK1A. J. Biol. Chem., 275, 2431–2438. [DOI] [PubMed] [Google Scholar]
- Kassis S., Melhuish,T., Annan,R.S., Chen,S.L., Lee,J.C., Livi,G.P. and Creasy,C.L. (2000) Saccharomyces cerevisiae Yak1p protein kinase autophosphorylates on tyrosine residues and phosphorylates myelin basic protein on a C-terminal serine residue. Biochem. J., 348, 263–272. [PMC free article] [PubMed] [Google Scholar]
- Kentrup H., Becker,W., Heukelbach,J., Wilmes,A., Schürmann,A., Huppertz,C., Kainulainen,H. and Joost,H.-G. (1996) Dyrk, a dual specificity protein kinase with unique structural features whose activity is dependent on tyrosine residues between subdomains VII and VIII. J. Biol. Chem., 271, 3488–3495. [DOI] [PubMed] [Google Scholar]
- Leder S., Weber,Y., Altafaj,X., Estivill,X., Joost,H.-G. and Becker,W. (1999) Cloning and characterization of DYRK1B, a novel member of the DYRK family of protein kinases. Biochem. Biophys. Res. Commun., 254, 474–479. [DOI] [PubMed] [Google Scholar]
- Lee K., Deng,X. and Friedman,E. (2000) Mirk protein kinase is a mitogen-activated protein kinase substrate that mediates survival of colon cancer cells. Cancer Res., 60, 3631–3637. [PubMed] [Google Scholar]
- Leupold U. (1970) Genetical methods for Schizosaccharomyces pombe. Methods Cell Physiol., 4, 169–177. [Google Scholar]
- Marks J. and Hyams,J.S. (1985) Localization of F-actin through the cell division cycle of S. pombe. Eur. J. Cell Biol., 39, 27–32. [Google Scholar]
- Mata J. and Nurse,P. (1997) tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell, 89, 939–949. [DOI] [PubMed] [Google Scholar]
- Mata J. and Nurse,P. (1998) Discovering the poles in yeast. Trends Cell Biol., 8, 163–167. [DOI] [PubMed] [Google Scholar]
- Matsusaka T., Hirata,D., Yanagida,M. and Toda,T. (1995) A novel protein kinase gene ssp1+ is required for alteration of growth polarity and actin localization in fission yeast. EMBO J., 14, 3325–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. (1990) nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J. Biol. Chem., 265, 10857–10864. [PubMed] [Google Scholar]
- Mitchison J.M. and Nurse,P. (1985) Growth in cell length in the fission yeast Schizosaccharomyces pombe. J. Cell Sci., 75, 357–376. [DOI] [PubMed] [Google Scholar]
- Miyata Y. and Nishida,E. (1999) Distantly related cousins of MAP kinase: biochemical properties and possible physiological functions. Biochem. Biophys. Res. Commun., 266, 291–295. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D. (1995) Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science, 268, 247–251. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar,A. and Nurse,P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol., 194, 795–823. [DOI] [PubMed] [Google Scholar]
- Nurse P. (1994) Fission yeast morphogenesis—posing the problems. Mol. Biol. Cell, 5, 613–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard J.E. and Kreis,T.E. (1996) CLIPs for organelle–microtubule interactions. Trends Cell Biol., 6, 178–183. [DOI] [PubMed] [Google Scholar]
- Rupes I., Jia,Z. and Young,P.G. (1999) Ssp1 promotes actin depolymerization and is involved in stress response and NETO control in fission yeast. Mol. Biol. Cell, 10, 1495–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K.L. and Nurse,P. (1998) Regulation of cell polarity by microtubules in fission yeast. J. Cell Biol., 142, 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman J.M. and St Johnston,D. (1999) Pattern formation in single cells. Trends Cell Biol., 9, M60–M64. [PubMed] [Google Scholar]
- Smith D.J. et al. (1997) Functional screening of 2 Mb of human chromosome 21q22.2 in transgenic mice implicates minibrain in learning defects associated with Down Syndrome. Nature Genet., 16, 28–36. [DOI] [PubMed] [Google Scholar]
- Sohrmann M., Fankhauser,C., Brodbeck,C. and Simanis,V. (1996) The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev., 10, 2707–2719. [DOI] [PubMed] [Google Scholar]
- Song W.-J. et al. (1996) Isolation of human and murine homologues of the Drosophila minibrain gene: human homologue maps to 21q22.2 in the Down Syndrome ‘critical region’. Genomics, 38, 331–339. [DOI] [PubMed] [Google Scholar]
- Souza G.M., Lu,S. and Kuspa,A. (1998) YakA, a protein kinase required for the transition from growth to development in Dictyostelium. Development, 125, 2291–2302. [DOI] [PubMed] [Google Scholar]
- Tejedor F., Zhu,X.R., Kaltenbach,E., Ackermann,A., Baumann,A., Canal,I., Heisenberg,M., Fischbach,K.F. and Pongs,O. (1995) minibrain: a new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron, 14, 287–301. [DOI] [PubMed] [Google Scholar]
- Verde F., Mata,J. and Nurse,P. (1995) Fission yeast cell morphogenesis: identification of new genes and analysis of their role during the cell cycle. J. Cell Biol., 131, 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Wiley,D.J. and Nurse,P. (1998) Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc. Natl Acad. Sci. USA, 95, 7526–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Sherwin,T., Sasse,R., MacRae,T.H., Baines,A.J. and Gull,K. (1989) Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci., 93, 491–500. [DOI] [PubMed] [Google Scholar]