Abstract
Continuous renewal of the epidermis and its appendages throughout life depends on the proliferation of a distinct population of cells called stem cells. We have used in situ retrovirus-mediated gene transfer to genetically mark cutaneous epithelial stem cells of adolescent mice, and have followed the fate of the marked progeny after at least 37 epidermal turnovers and five cycles of depilation-induced hair growth. Histological examination of serial sections of labeled pilosebaceous units demonstrated a complex cell lineage. In most instances, labeled cells were confined to one or more follicular compartments or solely to sebaceous glands. Labeled keratinocytes in interfollicular epidermis were confined to distinct columnar units representing epidermal proliferative units. The contribution of hair follicles to the epidermis was limited to a small rim of epidermis at the margin of the follicle, indicating that long term maintenance of interfollicular epidermis was independent of follicle-derived cells. Our results indicate the presence of multiple stem cells in cutaneous epithelium, some with restricted lineages in the absence of major injury.
Keywords: epidermis/hair follicle/lineage/sebaceous gland/stem cells
Introduction
Mammalian skin is composed of at least three differentiating epithelial compartments: the epidermis, the hair follicle and the associated glands (e.g. sebaceous glands). The growth of epidermis and sebaceous glands is continuous, while hair growth is cyclic. In epidermis, proliferation takes place in the basal layer, and terminally differentiated progeny move through the suprabasal layers to the tissue surface where they are shed as squames (Watt, 1998). Likewise, in the sebaceous gland, proliferation takes place in the periphery of the gland (the basal layer of the gland), and differentiating cells accumulate sebum as they move toward the sebaceous duct and finally rupture to release their contents into the pilosebaceous canal (Downing and Strauss, 1982). The hair follicle is a more complex structure, composed of at least seven distinct cell types organized in three compartments: an outer root sheath (ORS), an inner root sheath (IRS) and a hair shaft (Powell and Rogers, 1994). The lower two-thirds of the follicle undergo cycles of growth (anagen), regression (catagen) and rest (telogen) (Paus, 1998). During active growth, proliferation takes place in matrix cells in the hair bulb, with upward movement and differentiation to form the IRS and hair shaft at the center of the follicle (Hardy, 1992).
In all continually renewing tissues, a stem cell population is present to provide a source of differentiating cells (Lajtha, 1979; Hall and Watt, 1989). In its most basic form, a stem cell is one that has both the capacity for self-renewal, i.e. the ability to generate additional stem cells and thus be long lived, and the capacity to generate progeny that are fated to differentiate into at least one type of highly differentiated descendant (Lajtha, 1979; Morrison et al., 1997; Slack, 2000; Watt and Hogan, 2000). In mouse skin, an epithelial stem cell population is thought to localize to the bulge region of the hair follicle at the arrector pili muscle attachment site, a segment that does not undergo regression during the hair cycle (Lavker et al., 1991, 1993). In the classical experiments of Cotsarelis et al. (1990), stem cells were identified based primarily on their slow cycling nature, i.e. they retain labeled DNA precursors for prolonged periods. The bulge activation hypothesis that developed from this observation states that at the onset of anagen, multipotent stem cells in the bulge divide, and their daughter cells migrate to the base of the follicle and become matrix cells. The latter give rise to several lineages of terminally differentiated cells comprising different compartments of the hair follicle (Cotsarelis et al., 1990; Taylor et al., 2000). Moreover, the exclusive localization of label-retaining cells (LRCs) in the bulge region raises the possibility of long-term maintenance of the epidermis and sebaceous gland by the progeny of bulge stem cells (Lavker et al., 1993; Taylor et al., 2000). Direct evidence to support this hypothesis has been conflicting. Recently, Taylor et al. (2000) demonstrated that the progeny of LRCs in the bulge were found in both upper and lower portions of the follicles after a period of 10 week chase. However, when Morris and Potten (1999) followed LRCs in the bulge region after a longer chase period (more than a year), they noted that upon induction of anagen, LRCs were not among the first wave of proliferative cells and their daughter cells did not migrate to the matrix region during the completion of the 21 day hair cycle.
Cell lineage analysis of chimeric mouse skin had suggested a polyclonal origin for hair follicle formation during embryogenesis; however, the relative contribution of different clones to the several cell types comprising the hair follicle and its associated sebaceous gland was not demonstrated (Schmidt et al., 1987). More recently, using hair reconstitution assay with admixed populations of genetically labeled, cultured mouse keratinocytes, Kamimura et al. (1997) have shown a complex labeling pattern in reconstituted hair follicles. The discrete pattern of staining observed in distinct regions of the hair follicle suggested that multiple progenitor cells contribute to hair follicle formation. However, in these reconstituted follicles, sebaceous glands did not form and random orientation of follicles limited analysis of lineal relationships of the labeled cells. Previously, we described a procedure for in vivo transduction of mouse skin using retroviral vectors that resulted in integration and prolonged expression of the transgene (Ghazizadeh et al., 1999). In this study, it was noted that at 16 weeks post-transduction, transgene was expressed in various portions of the hair follicles, similar to that observed for labeled, reconstituted follicles (Kamimura et al., 1997). In the current study we have used in situ gene-marking of epithelial cells to analyze lineage patterns of cutaneous epithelium in adult mice for over half of the animals’ life expectancy, through multiple cycles of hair growth and epidermal turnovers. The study reported here marks the first lineage study of adult mouse skin epithelia. Our data confirm the multiclonal origin of hair follicles and provide strong evidence for the existence of long-lived progenitor populations in sebaceous gland and epidermis. These findings suggest that multiple classes of stem cells are involved in the continuous renewal of cutaneous epithelium during postnatal life.
Results
Retrovirus-mediated transduction of cutaneous epithelial stem cells
Recombinant retroviruses have been used to define lineal relationships among cells in several systems (Cepko et al., 1998). To study the lineage of cutaneous epithelial stem cells, retroviral vectors encoding Escherichia coli lacZ were used to mark adolescent mouse skin in situ. Initial β-galactosidase (β-gal) expression in epidermis was assessed by staining of cornified cells removed by tape stripping for β-gal activity (Ghazizadeh et al., 1999). While the turnover rate of mouse epidermis is estimated at ∼1 week (Potten, 1981), it has been difficult to determine turnover rates of pelage hair in adult mice due to loss of synchrony after the second hair cycle and the increased intervals between cycles with age (Sundberg and King, 1996). To ensure completion of several cycles of hair growth in transduced skin, the dorsal hair was depilated at 5, 12, 18, 24 and 36 weeks post-transduction. Staining of cornified cells removed by tape for β-gal activity the day after each depilation confirmed continued and faithful lacZ expression. More importantly, the general pattern of β-gal staining in the cornified cells was reproducible during the period of 31 weeks (Figure 1). While tape stripping is not a quantitative measure, the similar pattern of labeled cornified cells suggested that depilation and tape stripping have not resulted in an extensive migration and reorganization of labeled clones.
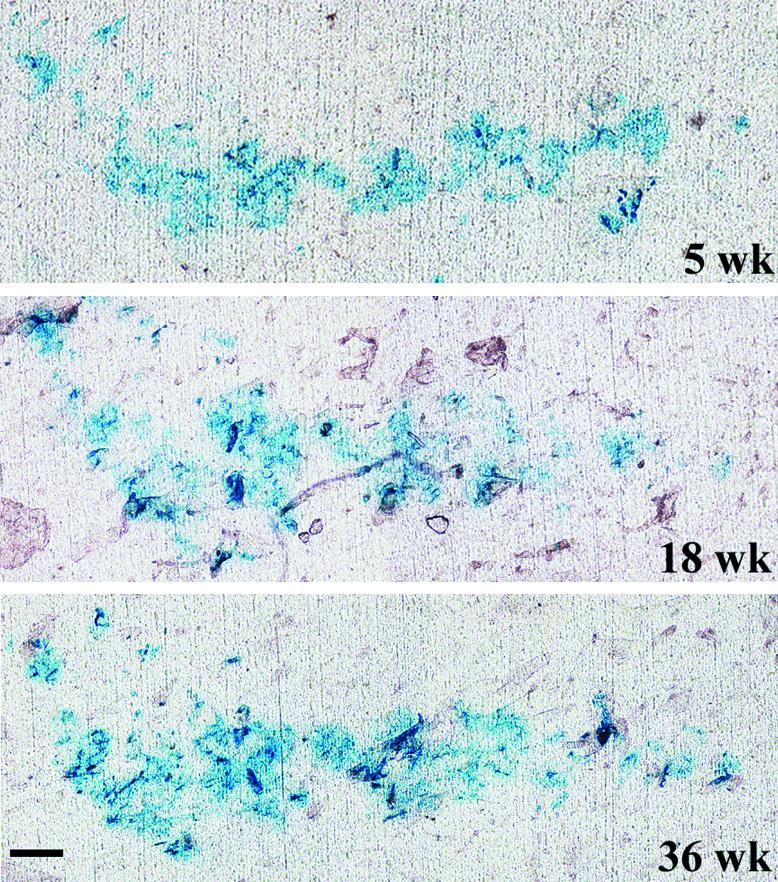
Fig. 1. Persistent expression of β-gal in epidermis. Dorsal skin of a MtnlacZ transgenic mouse was transduced with retroviruses encoding β-gal and, at the times indicated, β-gal expression was assessed by tape stripping and staining of adherent cornified cells with X-gal. Bar = 100 µm.
One week following the last depilation, transduced skin was biopsied and histochemical staining of serially cut cryosections revealed the lacZ-transduced cells and their progeny. All lacZ-positive cells (blue staining) were found in distinct clusters of various sizes (Figures 3–6). In all sections where individually stained keratinocytes were observed in a section, examination of adjacent sections revealed a direct connection to a cluster of labeled cells. The pattern and persistence of labeled cells for one-half of the animal’s lifespan indicated transduction of cells with the two main characteristics of a stem cell: longevity and generation of differentiated progeny.
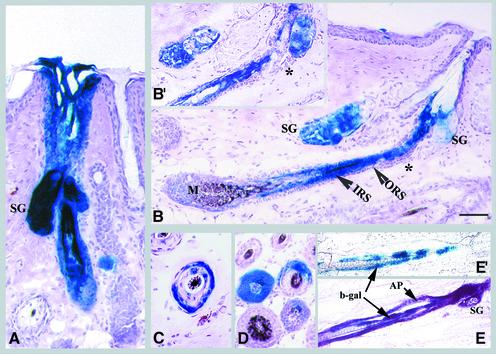
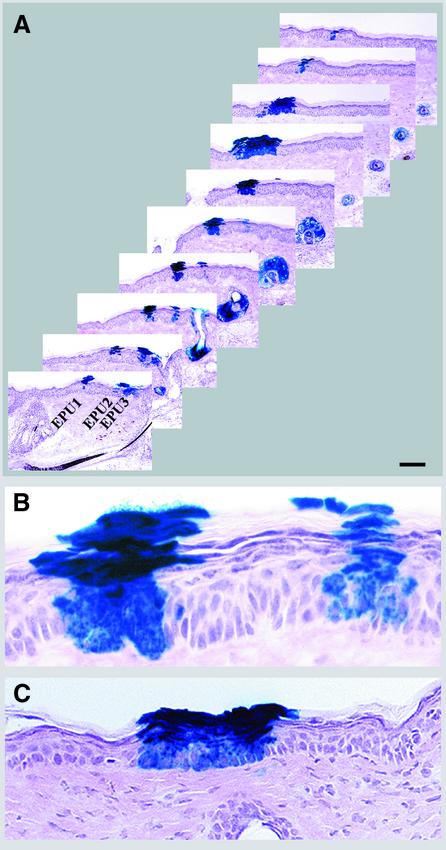
Fig. 3. Lineage analysis of hair follicles. (A–D) Mice were treated as described in Figure 2. Representative longitudinal sections are shown of (A) an anagen follicle with uniform labeling of all compartments, including sebaceous gland (SG) (the section shown does not show the full length of the follicle), (B) an anagen follicle with distinct labeling in the IRS and hair shaft in the absence of staining in the ORS, including the bulge (*) and (B′) an adjacent section of (B) demonstrating the absence of β-gal-positive cells in the bulge (*). Note that labeled cells in (B) are traced back to hair matrix (M). The lower panels show follicles with β-gal-positive cells confined mainly to the ORS (C) or to a portion of follicular compartments (D). (E and E′) Rag-2 mice were transduced with a mixture of two viruses encoding PLAP and β-gal. Sections were stained for PLAP (AP, purple) and β-gal (blue). (E′) is an adjacent section of (E) that was stained with X-gal alone to demonstrate confinement of β-gal-positive cells to one compartment. Scale bar: 60 µm (A and C); 100 µm (B and D); 120 µm (E and E′).
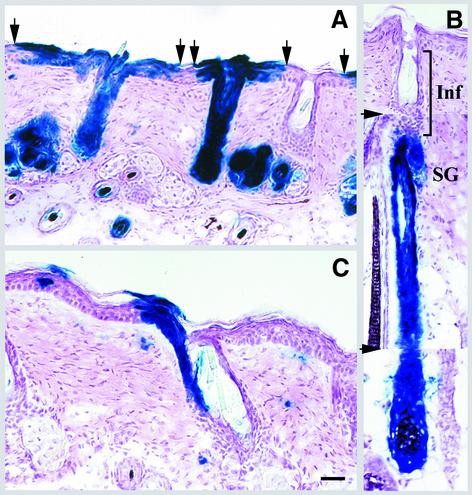
Fig. 6. Re-epithelialization of abraded epidermis by epithelial cells in infundibulum. Transduced skin was abraded for a second time at 36 weeks post-transduction, and 6 weeks later serial sections of skin were analyzed. (A) The increased size of follicular extensions indicates re-epithelialization by follicle-derived cells. Arrows denote the boundary of follicular extensions. (B) A composite of three serial sections to illustrate labeling of only the lower portion of a follicle. The arrowheads denote the photo splice sites. The non-labeled infundibulum (Inf) is shown. (C) The upper portion of a follicle labeled in one side of infundibulum demonstrates extension of labeled cells to epidermis. The isolated X-gal-positive cells in the dermis are transduced fibroblasts. Scale bar: 60 µm (A–B); 40 µm (C).
Lineage analysis of mouse pilosebaceous units
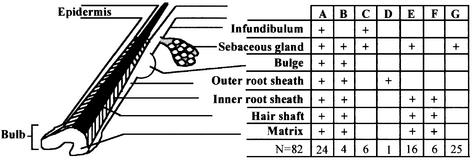
Lineage analysis was carried out by examining serially sectioned tissues. A total of 82 labeled pilosebaceous units derived from three different mice (10 months old) were analyzed, and the various types of labeled follicles were scored as summarized in Figure 2. Approximately 30% of labeled pilosebaceous units were uniformly labeled in all components, including IRS, ORS, hair shaft, sebaceous glands and associated epidermal extension (Figure 3A). In a large fraction of labeled follicles (70%), however, labeled cells were confined to one or more of the follicular compartments, e.g. to the IRS and sebaceous gland (Figure 3B), to the ORS (Figure 3C) or to the sebaceous gland (Figure 4). A follicle distinctly labeled in the IRS and hair shaft is shown in Figure 3B. In this follicle, labeled epithelial cells were traced back to the hair matrix region in the base of the follicle. No lacZ-positive cell was observed in the ORS of that follicle (Figure 3B and B′). In some follicles, lacZ-positive cells were confined to a portion of a follicular compartment, giving a crescent appearance in cross-section (Figure 3D). Furthermore, differential β-gal labeling was observed in the upper and lower portions of some labeled follicles. While some follicles were labeled only in the lower portions below the infundibulum, others showed labeling confined to the infundibulum (Figure 6). These data indicate that multiple progenitor cells are involved in anagen follicle formation and that cell lineages in the upper and lower portions of hair follicles are distinct.
Fig. 2. Distribution of lacZ-positive cells in various compartments of labeled pilosebaceous units. Serial sections of skin of transduced mice following five cycles of induced hair growth, at 37 weeks post-transduction, were analyzed by X-gal staining and the distribution of lacZ-positive clusters in the various compartments of 82 pilosebaceous units was noted. ‘+’ indicates partial or uniform labeling of cells in that compartment. The number of labeled pilosebaceous units in each category is shown in the bottom row.
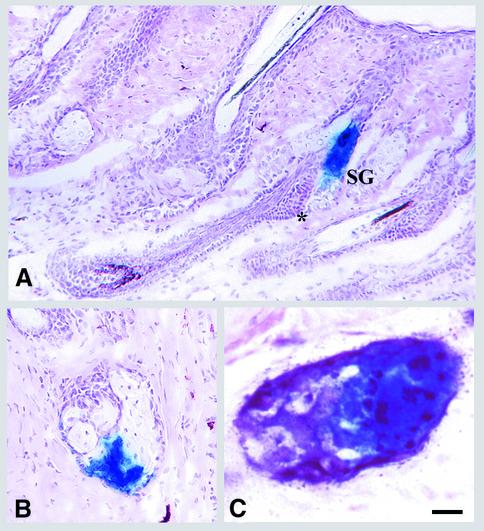
Fig. 4. Presence of long-lived progenitor cells in sebaceous glands. The experiment was the same as Figure 2. In (A), representative longitudinal sections of follicles with β-gal-positive cells confined solely to sebaceous glands are shown. Note the absence of β-gal-positive cells in the bulge (*). Representative cross-sections of a partially stained sebaceous gland (B) or a transduced gland with distinct clusters of β-gal- (blue) and alkaline phosphatase-positive (purple) cells (C) are shown. Scale bars: 50 µm (A); 35 µm (B); 5 µm (C).
In 30% of labeled pilosebaceous units, β-gal-positive cells were confined solely to sebaceous glands (Figure 4). Although homogeneous labeling of sebocytes in the sebaceous gland was observed in some glands (Figure 4A), in others β-gal-positive cells were closely grouped in a portion of the gland (Figure 4B and C). As shown in Figure 4A, no labeled cell was observed in the bulge area or other parts of the hair follicle. We could not comment on the presence of β-gal-labeled cells in the sebaceous duct due to the apparent lack of this structure in mouse skin. The presence of partially labeled sebaceous glands suggests the presence of multiple proliferative units within the gland, each composed of a committed progenitor cell and its differentiated progeny of mature sebocytes. Based on an average transit time of 14 days for sebocytes (Downing and Strauss, 1982), the labeled progenitor sebocytes must have persisted for >18 turnovers. This is the first demonstration of long-lived progenitor cells in sebaceous glands maintaining a separate cell lineage.
As noted above, 29% of the labeled follicles were labeled uniformly in all compartments. A homogeneously labeled follicle could have arisen from a single pluripotent stem cell, or, in light of the partial labeling seen in most follicles, could have been derived from multiple stem cells in the follicle, each transduced with the retroviral vector. The pronounced proliferative response of follicles to abrasion (Argyris, 1976; Ghazizadeh et al., 1999) increases the likelihood of gene transfer to all dividing cells in a given follicle. To examine the possibility of transducing multiple stem cells in a follicle, a mixture of two recombinant retroviruses encoding lacZ or plap (human placental alkaline phosphatase) was used to transduce mouse skin. Fourteen weeks later, following three depilation-induced hair cycles, transduced tissue was stained for both β-gal and PLAP activities. Three types of transduced cells were evident after examination of tape-stripped cornified cells: those expressing β-gal (11%), PLAP (54%) or both markers (35%) (n = 167). The higher ratio of PLAP-positive cells is reflective of the higher titer of plap virus (2.5-fold) in comparison with lacZ virus. Histological examination of transduced skin stained for both β-gal and PLAP activities revealed clusters of PLAP-positive, β-gal-positive and doubly positive cells where both genes were expressed in the same cell. The presence of doubly labeled cells indicated the integration of multiple viruses in a transduced cell. In addition, no random ‘salt and pepper’ mixing of differentially labeled cells was observed in the labeled clusters. Fully labeled follicles or sebaceous glands were evident, which contained two types of labeled clusters, each localized to different follicular compartments (Figure 3E and E′) or to distinct portions of a sebaceous gland (Figure 4C). These data confirmed a multiclonal origin of hair follicle and sebaceous gland.
Lineage analysis of epidermal keratinocytes
In mouse epidermis, an epidermal stem cell and its progeny are organized in a spatially distinct unit called an epidermal proliferative unit (EPU) (Allen and Potten, 1974). Examination of transduced epidermis demonstrated distinct columns of β-gal-expressing keratinocytes extending from the basal layer to the uppermost differentiated layers, indicative of EPUs (Figure 5A). In hair-bearing skin, it has been suggested that cells in the hair follicle contribute continuously to the normal maintenance of epidermis (Taylor et al., 2000). We therefore expected to trace all labeled EPUs to labeled follicles. However, analysis of 42 labeled EPUs demonstrated that whereas about half of the labeled EPUs were traced back to labeled follicles [Figure 5A (EPU3) and C], the other half were independent of follicles [Figure 5A (EPU1 and 2) and B]. Some of the labeled EPUs were isolated and located at a large distance from labeled follicles (data not shown). Longitudinal sections cut through the labeled follicles demonstrated a two to four cell extension of stratified epithelium of infundibulum into the epidermis [Figures 3A and 5A (EPU3)]. In serial sections, these extensions appeared as EPUs comparable in size and organization to interfollicular EPUs (Figure 5C; note that Figures 3A and 5C are representative serial sections of the same follicle). The presence of β-gal-expressing interfollicular EPUs maintained for ∼37 epidermal turnovers suggested that there are long-lived, lineage-restricted progenitors in epidermis that are sufficient to maintain tissue renewal in the absence of major injury.
Fig. 5. Lineage analysis of mouse epidermis. (A–C) The experiment was the same as Figure 2. (A) Every third section of serially cut transduced epidermis (extending over a 210 µm thickness) is presented to reveal the origin of EPUs (follicular or interfollicular). Note the different sizes of EPU1 and 2. EPU3 is an extension of follicular infundibulum. (B) Higher magnification of the fifth section of interfollicular epidermis shown in (A). (C) Follicular extensions of the follicle shown in Figure 3A appear as an EPU in adjacent sections. The distance between sections shown here and that shown in Figure 3A is 42 µm. Scale bar: 150 µm (A); 25 µm (B); 35 µm (C).
It has been known for some time (Al-Barwari and Potten, 1976), and recently confirmed (Taylor et al., 2000), that following an epidermal injury, follicular cells repopulate the denuded epidermis. Since dermabrasion was used to induce the initial hyperplasia required for retrovirus-mediated gene transfer, the likely sources of targeted epidermis were follicle-derived cells that had migrated to the epidermis within 3 days post-abrasion. To test this hypothesis, the dorsal skin of transduced mice was abraded for the second time at 36 weeks following the initial transduction, depilated at week 41 to induce anagen, and 1 week later the pattern of lacZ-positive cells in re-epithelialized epidermis was analyzed. At this time, epidermis has turned over at least six times and hair follicles have entered a second cycle after the wounding. As shown in Figure 6A, epidermis was now re-epithelialized by cells emerging from hair follicles, as indicated by a zone of labeled epidermis surrounding each labeled follicle. The number of lacZ-positive cells extending out of the follicle increased from two to four cells to seven to 12 cells at each side of the follicle (compare Figure 6A with 3A, or with the third section in 5A). Furthermore, all labeled EPUs examined in this tissue were traced back to labeled follicles (data not shown), indicating repopulation of interfollicular epidermis by follicle-derived cells. In addition, migration of transduced follicular cells into epidermis indicated that expression of lacZ had not perturbed stem cell function and pluripotency. To determine the origin of follicle-derived cells, serial sections from follicles distinctly labeled in the upper or lower portions were examined. Figure 6B and C is representative of follicles distinctly labeled in the lower portions below the infundibulum and those labeled in the infundibulum, respectively. It was noted that in the latter follicle, the infundibulum and contiguous epidermis are labeled only on one side. The sebaceous gland in this follicle was also labeled; however, no labeled cell was observed in the lower portion of this follicle (data not shown). In both types of labeled follicles, the pattern of labeling in epidermis was contiguous with the infundibulum, suggesting that upon loss of interfollicular epidermis, keratinocytes in the infundibulum migrate out and establish new EPUs.
Discussion
Cutaneous epithelium is a continuously renewing tissue consisting of at least three well defined histological structures including epidermis, hair follicles and sebaceous glands. A central question in cutaneous biology is the mechanism of renewal and maintenance of this tissue throughout postnatal life. Either there is a pluripotent stem cell population that gives rise to all these differentiated structures, or there are distinct stem cell classes with more restricted lineage. In this study, we employed the term ‘stem cells’ to describe self-renewing cells that can produce at least one type of highly differentiated descendant. Transient amplifying cells have the capacity to generate differentiated cells, but have a limited life expectancy (Lajtha, 1979; Hall and Watt, 1989; Watt and Hogan, 2000). In the absence of biochemical or genetic markers to define stem cells by features other than their replicative behavior, it is not possible to use a more precise set of terms for these different classes of renewing cells.
In the present study we have transduced mouse skin in situ with a β-gal-encoding vector, and 37 weeks later, after five cycles of induced hair growth, analyzed the distribution pattern of transduced cells and their progeny. Pilosebaceous units were labeled in the following ways: uniformly labeled throughout (29%), partially labeled follicles (40%) and those labeled solely in the sebaceous gland (31%). In interfollicular epidermis, 43% of the 42 labeled columns analyzed could not be traced back to a labeled follicle. Some labeled EPUs were located at a large distance from labeled follicles. Several conclusions can be drawn from these results. First, based on the presence of long-lived β-gal-positive cells (about half of the life expectancy of the animal) and on the presence of labeled clusters (labeled lineages), we conclude that the initial exposure to retrovirus had indeed transduced stem cells. Secondly, the presence of pilosebaceous units with singly labeled IRS, ORS or sebaceous gland indicates that regeneration of these units involves multiple stem cells, at least one for each compartment. Thirdly, the fact that in some cases only a portion of follicle or sebaceous gland was labeled indicates that in these instances more than one precursor cell has contributed to the formation of that structure.
The initial dermabrasion was required for successful transduction not only to induce proliferation of stem cells, but also to allow access to the progenitor population (Ghazizadeh et al., 1999). Our analysis was performed several months after the initial injury, when the tissue is likely to be normalized. Indeed, the similar staining patterns in cornified cells removed by repeated tape stripping over a period of 36 weeks indicated a lack of significant reorganization or migration of labeled clusters after 5 weeks post-wounding. Furthermore, these similar staining patterns indicate that depilation and tape stripping did not result in severe damage and reorganization of labeled cells.
It is unlikely that the observed pattern of labeled clusters in pilosebaceous units was a consequence of restricted expression of the reporter gene (lacZ or plap). It is known that the site of retroviral integration can affect the level of expression of its encoded genes (Emerman and Temin, 1984); however, it is unlikely that multiple, random, independent integration events could have generated the repeatable labeling patterns seen in partially labeled follicles. Furthermore, no partially labeled EPUs were detected. Had the site of integration been the underlying cause of partially labeled follicles, one would have expected to see partially labeled EPUs as well (i.e. labeling of either basal or suprabasal cells in epidermis). It is also unlikely that expression from the retroviral promoter was cell specific, as this promoter is not known to be expressed in a cell-type-specific manner and, indeed, labeling was seen in all compartments of the skin, including dermal fibroblasts, in this study. Promoter inactivation is also an unlikely event as repeated tape stripping of transduced skin areas revealed no loss of staining over a period of 36 weeks (Figure 1). Promoter shut-off, when it has been observed, occurs early, within 4–6 weeks after transplantation of transduced cells, and is an irreversible, all-or-none phenomenon (Palmer et al., 1991; Fenjves et al., 1996; Choate and Khavari, 1997). Furthermore, a detailed clonal analysis of human keratinocytes transduced with the same vector indicated no loss of transgene expression throughout 40 weeks in vivo (Kolodka et al., 1998).
The polyclonal origin of the hair follicle has been suggested by analysis of chimeric mouse skin and retrovirus-labeled follicles reconstituted from cultured newborn keratinocytes and follicular fibroblasts (Schmidt et al., 1987; Kamimura et al., 1997). As noted above, 29% of the labeled pilosebaceous units were labeled uniformly in all compartments. A homogeneously labeled follicle observed could have arisen from a single pluripotent stem cell, or, in light of the partial labeling seen in most follicles, from multiple stem cells in the follicle, each transduced with the retroviral vector. Experimental verification of multiple transduction events in uniformly labeled follicles was evident when uniformly labeled follicles were observed with both β-gal- and PLAP-positive clusters of cells in different follicular compartments.
Another issue raised by our results was the lineage of the upper and lower portions of the ORS. The differences in morphology, differentiation program and kinetics of ORS regeneration in the upper and lower portions of the follicle (Coulombe et al., 1989) suggest a complex lineage for the ORS. Our analysis of follicles with ORS labeling confined to either the upper or lower portions is consistent with the presence of distinct lineages for each portion. Following the removal of epidermis by wounding, re-epithelialization occurs by cells migrating from the upper ORS cells in the infundibulum (Al-Barwari and Potten, 1976; Taylor et al., 2000; Figure 6). Our data showed that following abrasion-induced re-epithelialization, the pattern of β-gal staining in contiguous epidermis followed that of the infundibulum, regardless of the staining pattern in the lower portions of the follicle (Figure 6B and C).
Recently, Taylor et al. (2000) showed, through a double metabolic labeling procedure in newborn mice or wounded skin, that labeled cells in the infundibulum migrated outward to reside in interfollicular areas. It was suggested that normal maintenance of interfollicular epidermis is achieved by a pool of follicular cells. It is important to note that in our study transduction was performed 3 days after dermabrasion and, therefore, all labeled epidermal cells seen at later times were follicular in origin. Had there been continuous repopulation of epidermis from neighboring follicles, all labeled EPUs would have been connected to labeled follicles. Half of the labeled EPUs were free-standing. The possibility of follicle-derived progenitors migrating within epidermis some distance away from their site of origin is unlikely, since some EPUs were found at a large distance from labeled follicles or surrounded by non-labeled follicles. Since every follicular unit has its own stem cells, it is difficult to envisage that the progeny of stem cells from one unit migrate beyond the boundaries of that unit. Our results indicate that while epidermal cells could originate from follicles under certain conditions, i.e. severe epidermal injury, there is not always a continuous repopulation of interfollicular epidermis from follicles under steady-state conditions.
Re-epithelialization of epidermis by follicle-derived cells demonstrates the plasticity and developmental flexibility of epithelial cells in a wounding environment (Reynolds and Jahoda, 1994). Transdifferentiation of adult epidermal keratinocytes to form hair follicles after induction by dermal papilla has also been demonstrated (Reynolds and Jahoda, 1992; Ferraris et al., 1997). Recently, de novo hair follicle formation from epidermal keratinocytes in mice expressing a stabilized β-catenin controlled by an epidermal promoter was demonstrated (Gat et al., 1998). These experiments indicate that even though the fate decision to become epidermis, hair follicle or sebaceous gland is made during embryonic life (Hardy, 1992), epithelial cells in postnatal skin retain their ability to respond to environmental signals by changing to another lineage. Stable proliferation and maintenance of a specific lineage in epidermis, sebaceous gland or hair follicle might be a function of signals received from their microenvironment. Many signaling molecules are implicated in the regulation of various aspects of hair morphogenesis (reviewed in Oro and Scott, 1998; Fuchs and Segre, 2000). A role for these and other molecules in the establishment or refinement of various compartments of cutaneous epithelium remains to be elucidated.
In summary, our findings provide evidence for the existence of multiple stem cells with restricted lineage in cutaneous epithelium. The stem cells and their respective lineal descendants may be organized into structural– proliferative units similar to those described for other epithelia (for a review see Slack, 2000). Our data also suggest that the loss of a stem cell in one proliferative unit is rapidly replaced by stem cells in the adjacent unit. The multipotential nature and developmental flexibility of adult cutaneous epithelial cells have been well documented (Reynolds and Jahoda, 1994), and reflect the high demands that nature has placed on a tissue with a relatively invariant architecture.
Materials and methods
Viral vectors
MFG-based retroviral vectors encoding E.coli lacZ under the control of the 5′ long terminal repeat (Danos and Mulligan, 1988) were pseudotyped with vesicular somatitis virus envelope protein (Burns et al., 1993; Ghazizadeh et al., 1999). Concentrated virus preparations with titers of 1 × 109 β-gal-positive colony-forming units (c.f.u.)/ml, as determined on NIH 3T3 cells, were used for in vivo transduction. In some experiments, a pseudotyped MFG-based vector encoding plap was mixed with lacZ-encoding viruses prior to concentration. The titer of each virus in the mixture was 7 × 108 β-gal- and 1.8 × 109 alkaline phosphatase-positive c.f.u./ml.
Animals and in vivo transduction
Experiments were performed on 7-week-old MTnlacZ transgenic mice (Jackson Labs) expressing lacZ under the control of the metallothionein 1 promoter (Rhim et al., 1994). This promoter is active in liver, thereby inducing tolerance to β-gal protein. Because of immunogenicity of PLAP in transgenic mice, Rag-2 immunodeficient mice (Taconic) were transduced with the mixture of lacZ- and plap-encoding viruses. At 7 weeks of age, hair follicles are in telogen. To induce replication of target cells, dorsal skin of mice was depilated using a depilatory agent (Nair; Carter-Wallace Inc.) and dermabraded (Ghazizadeh et al., 1999). On day 3 post-abrasion, 20 µl (containing 2 × 107 c.f.u.) of retrovirus were injected into the hyperproliferative skin. Animal studies were performed in accordance with institutional guidelines set forth by the State University of New York.
Detection of transgene expression in transduced skin
To assess β-gal expression in transduced skin without an excisional biopsy, cornified cells on the surface of depilated epidermis were removed by tape stripping and analyzed by 4-chromo-5-bromo-3-indolyl-β-D-galactosidase (X-gal) staining of the tape (Ghazizadeh et al., 1999). For histological examination, skin specimens were snap-frozen in OCT, and 7-µm-thick serial sections were stained with X-gal for 1 h and counterstained with hematoxylin–eosin (H&E). When viral mixtures were used, following X-gal staining, sections were washed and heated at 68°C for 45 min to inhibit endogenous PLAP activity, and stained for PLAP activity by incubation in X-P reaction mix (1 mg/ml 5-bromo-4-chloro-3-indolyl-phosphate and 1 mg/ml nitroblue tetrazolium in 100 mM Tris–HCl pH 9.5, 100 mM NaCl, 50 mM MgCl2) for 20 min at room temperature (Fekete and Cepko, 1993). Sections were washed in phosphate-buffered saline containing 20 mM EDTA and counterstained with Schiff reagent (Sigma).
Acknowledgments
Acknowledgements
We are grateful to Drs Marcia Simon and Raphael Kopan for critical reading of the manuscript and useful suggestions, and to Robin Harrington and Ning Lin for technical assistance. This research was supported by National Institute of Health Grants DEO4511 (to L.B.T.), AR02100 and AR08390 (to S.G.).
References
- Al-Barwari S.E. and Potten,C.S. (1976) Regeneration and dose–response characteristics of irradiated mouse dorsal epidermal cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med., 30, 201–216. [DOI] [PubMed] [Google Scholar]
- Allen T.D. and Potten,C.S. (1974) Fine-structural identification and organization of the epidermal proliferative unit. J. Cell Sci., 15, 291–319. [DOI] [PubMed] [Google Scholar]
- Argyris T.S. (1976) Kinetics of epidermal production during epidermal regeneration following abrasion in mice. Am. J. Pathol., 83, 329–340. [PMC free article] [PubMed] [Google Scholar]
- Burns J.C., Friedmann,T., Driever,W., Burrascano,M. and Yee,J.K. (1993) Vesicular somatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and non-mammalian cells. Proc. Natl Acad. Sci. USA, 90, 8033–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C.L., Ryder,E., Austin,C., Golden,J., Fields-Berry,S. and Lin,J. (1998). Lineage analysis using retroviral vectors. Methods, 14, 393–406. [DOI] [PubMed] [Google Scholar]
- Choate K.A. and Khavari,P.A. (1997) Sustainability of keratinocyte gene transfer and cell survival in vivo. Hum. Gene Ther., 8, 895–901. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G., Sun,T.-T. and Lavker,R.M. (1990) Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell, 61, 1329–1337. [DOI] [PubMed] [Google Scholar]
- Coulombe P.A., Kopan,R. and Fuchs,E. (1989) Expression of keratin K14 in the epidermis and hair follicle: insights into complex programs of differentiation. J. Cell Biol., 109, 2295–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O. and Mulligan,R.C. (1988) Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc. Natl Acad. Sci. USA, 85, 6460–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing D.T. and Strauss,J.S. (1982) On the mechanism of sebaceous secretion. Arch. Dermatol. Res., 272, 343–349. [DOI] [PubMed] [Google Scholar]
- Emerman M. and Temin,H.M. (1984) Genes with promoters in retrovirus vectors can be independently suppressed by an epigenic mechanism. Cell, 39, 449–467. [PubMed] [Google Scholar]
- Fekete D.M. and Cepko,C.L. (1993) Replication-competent retroviral vectors encoding alkaline phosphatase reveal spatial restriction of viral gene expression/transduction in the chick embryo. Mol. Cell. Biol., 13, 2604–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenjves E.S., Yao,S.-N., Kurachi,K. and Taichman,L.B. (1996) Analysis of loss of expression of a retrovirus-transduced gene in grafted human keratinocytes. J. Invest. Dermatol., 106, 576–578. [DOI] [PubMed] [Google Scholar]
- Ferraris C., Bernard,B.A. and Dhouailly,D. (1997) Adult epidermal keratinocytes are endowed with pilosebaceous forming abilities. Int. J. Dev. Biol., 41, 491–498. [PubMed] [Google Scholar]
- Fuchs E. and Segre,J.A. (2000) Stem cells: a new lease on life. Cell, 100, 143–155. [DOI] [PubMed] [Google Scholar]
- Gat U., DasGupta,R., Degenstein,L. and Fuchs,E. (1998). De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell, 95, 605–614. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S., Harrington,R. and Taichman,L.B. (1999) In vivo transduction of mouse epidermis with recombinant retroviral vectors: implications for cutaneous gene therapy. Gene Ther., 6, 1267–1275. [DOI] [PubMed] [Google Scholar]
- Hall P.A. and Watt,F.M. (1989) Stem cells: the generation and maintenance of cellular diversity. Development, 106, 619–633. [DOI] [PubMed] [Google Scholar]
- Hardy M.H. (1992) The secret life of the hair follicle. Trends Genet., 8, 55–61. [DOI] [PubMed] [Google Scholar]
- Kamimura J., Lee,D., Baden,H.P., Brissette,J. and Dotto,G.P. (1997) Primary mouse keratinocytes cultures contain hair follicle progenitor cells with multiple differentation potential. J. Invest. Dermatol., 109, 534–540. [DOI] [PubMed] [Google Scholar]
- Kolodka T.M., Garlick,J.A. and Taichman,L.B. (1998) Evidence for keratinocyte stem cells in vitro: long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc. Natl Acad. Sci. USA, 95, 4356–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajtha L.G. (1979) Stem cell concepts. Differentiation, 14, 23–34. [DOI] [PubMed] [Google Scholar]
- Lavker R.M., Cotsarelis,G., Wei,Z.G. and Sun,T.T. (1991) Stem cells of pelage, vibrissae, and eyelash follicles: the hair cycle and tumor formation. Ann. NY Acad. Sci., 642, 214–225. [DOI] [PubMed] [Google Scholar]
- Lavker R.M., Miller,S., Wilson,C., Cotsarelis,G., Wei,Z.G., Yang,J.S. and Sun,T.T. (1993) Hair follicle stem cells: their location, role in hair cycle, and involvement in skin tumor formation. J. Invest. Dermatol., 101, 16S–26S. [DOI] [PubMed] [Google Scholar]
- Morris R.J. and Potten,C.S. (1999) Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J. Invest. Dermatol., 112, 470–475. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Shah,N.M. and Anderson,D.J. (1997) Regulatory mechanisms in stem cell biology. Cell, 88, 287–298. [DOI] [PubMed] [Google Scholar]
- Oro A.E. and Scott,M.P. (1998) Splitting hairs: dissecting roles of signaling systems in epidermal development. Cell, 95, 575–578. [DOI] [PubMed] [Google Scholar]
- Palmer T.D., Rosman,G.L., Osborne,W.R. and Miller,A.D. (1991) Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc. Natl Acad. Sci. USA, 88, 1330–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R. (1998) Principles of hair cycle control. J. Dermatol., 25, 793–802. [DOI] [PubMed] [Google Scholar]
- Potten C.S. (1981) Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int. Rev. Cytol., 69, 271–318. [DOI] [PubMed] [Google Scholar]
- Powell B.C. and Rogers,G.E. (1994) Differentiation in hard keratin tissues: hair and related structures. In Leigh,I., Lane,B. and Watt,F. (eds), The Keratinocyte Handbook. Cambridge University Press, Cambridge, UK, pp. 401–436.
- Reynolds A.J. and Jahoda,C.A. (1992) Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development, 115, 587–593. [DOI] [PubMed] [Google Scholar]
- Reynolds A.J. and Jahoda,C.A. (1994) Hair follicle stem cells: characteristics and possible significance. Skin Pharmacol., 7, 16–19. [DOI] [PubMed] [Google Scholar]
- Rhim J.A., Sandgren,E.P., Degen,J.L., Palmiter,R.D. and Brinster,R.L. (1994) Replacement of diseased mouse liver by hepatic cell transplantation. Science, 263, 1149–1152. [DOI] [PubMed] [Google Scholar]
- Schmidt G.H., Blount,M.A. and Ponder,B.A.J. (1987) Immunochemical demonstration of the clonal organization of chimeric mouse epidermis. Development, 100, 535–541. [DOI] [PubMed] [Google Scholar]
- Slack J.M.W. (2000) Stem cells in epithelial tissues. Science, 287, 1431–1433. [DOI] [PubMed] [Google Scholar]
- Sundberg J.P. and King,L.E.,Jr (1996) Mouse mutations as animal models and biomedical tools for dermatological research. J. Invest. Dermatol., 106, 368–376. [DOI] [PubMed] [Google Scholar]
- Taylor G., Lehrer,M.S., Jensen,P.J., Sun,T.T. and Lavker,R.M. (2000) Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell, 102, 451–461. [DOI] [PubMed] [Google Scholar]
- Watt F.M. (1998) Epidermal stem cells: marker, patterning and the control of stem cell fate. Philos. Trans. R. Soc. Lond. B Biol. Sci., 353, 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F.M. and Hogan,B.L. (2000) Out of Eden: stem cells and their niches. Science, 287, 1427–1430. [DOI] [PubMed] [Google Scholar]