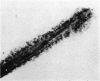
Abstract
A reproducible in vitro test was developed to quantitatively study the adhesion of human eosinophils to Wuchereria bancrofti infective larvae. Eosinophils, regardless of the donor, selectively adhered to the larvae in the presence of immune serum. The reaction reached a maximum by 90 minutes at room temperature and remained unchanged up to 6 hours. The adherent eosinophils, however, did not induce any apparent morphologic change in the larvae.
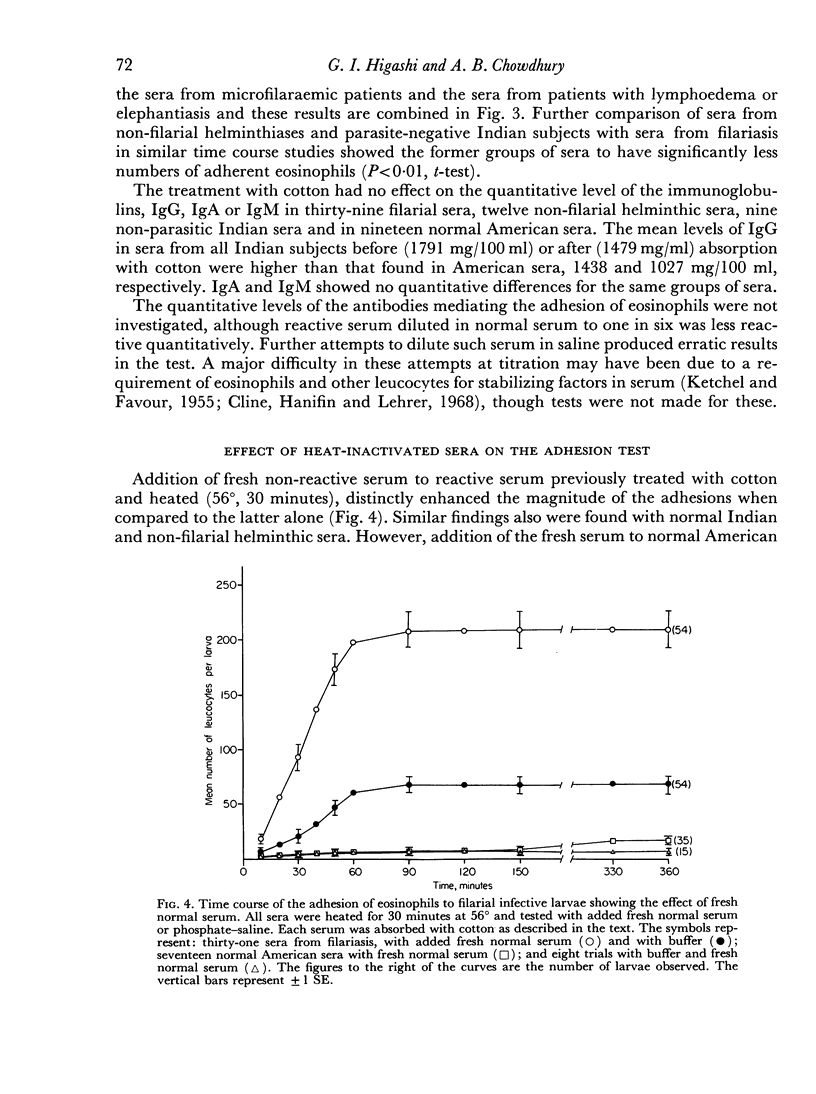
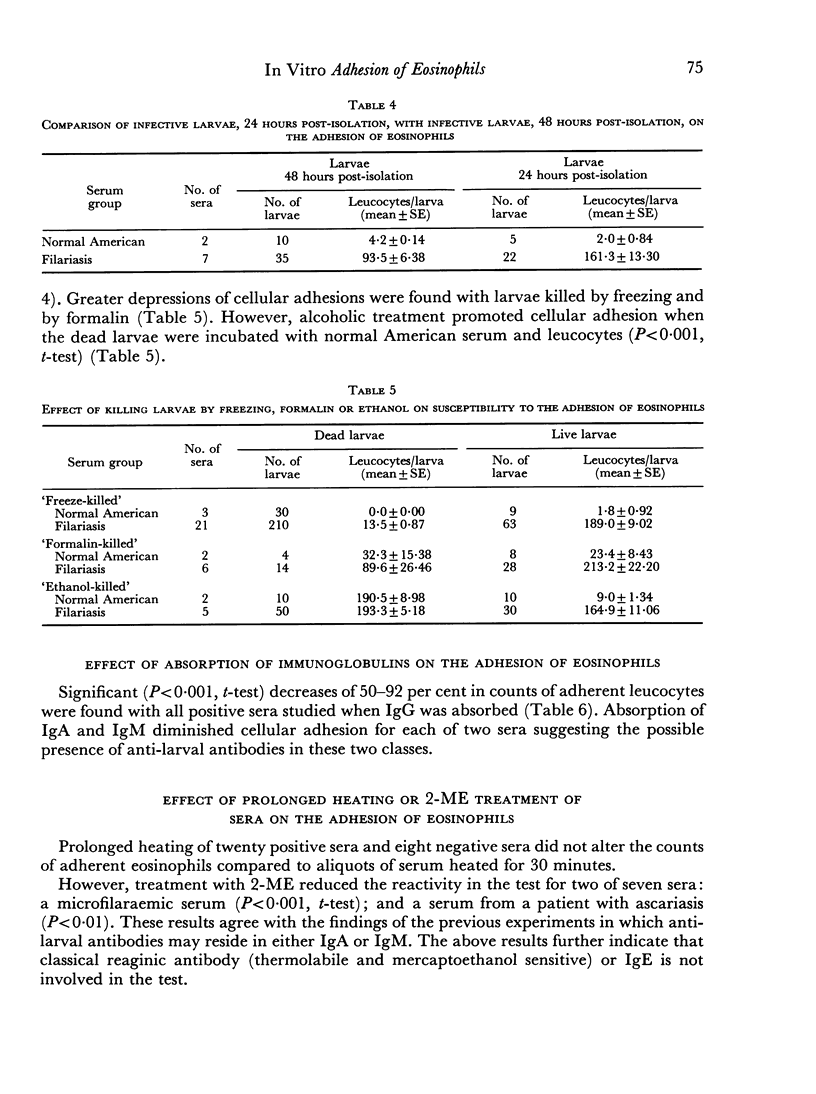
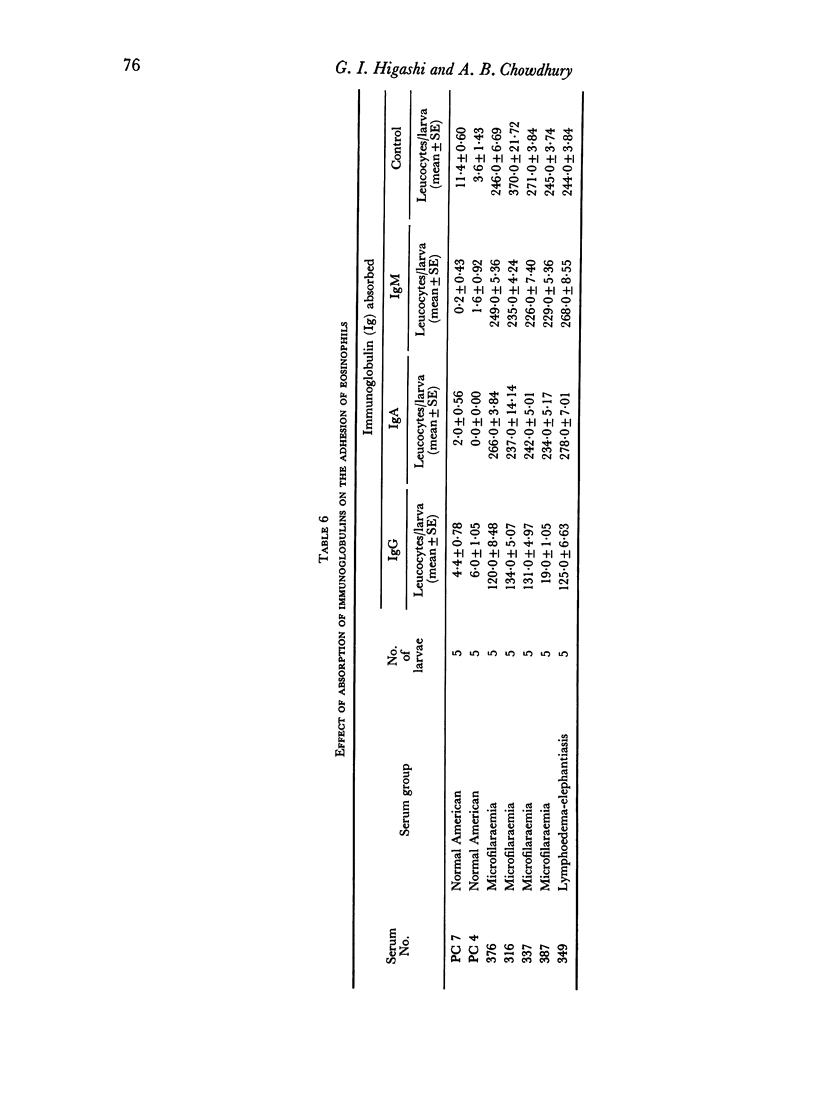
The phenomenon appeared to require, primarily, IgG anti-larval antibodies. Heat-inactivation of the serum did not prevent the reaction from occurring, although addition of fresh normal serum enhanced the intensity of adhesion.
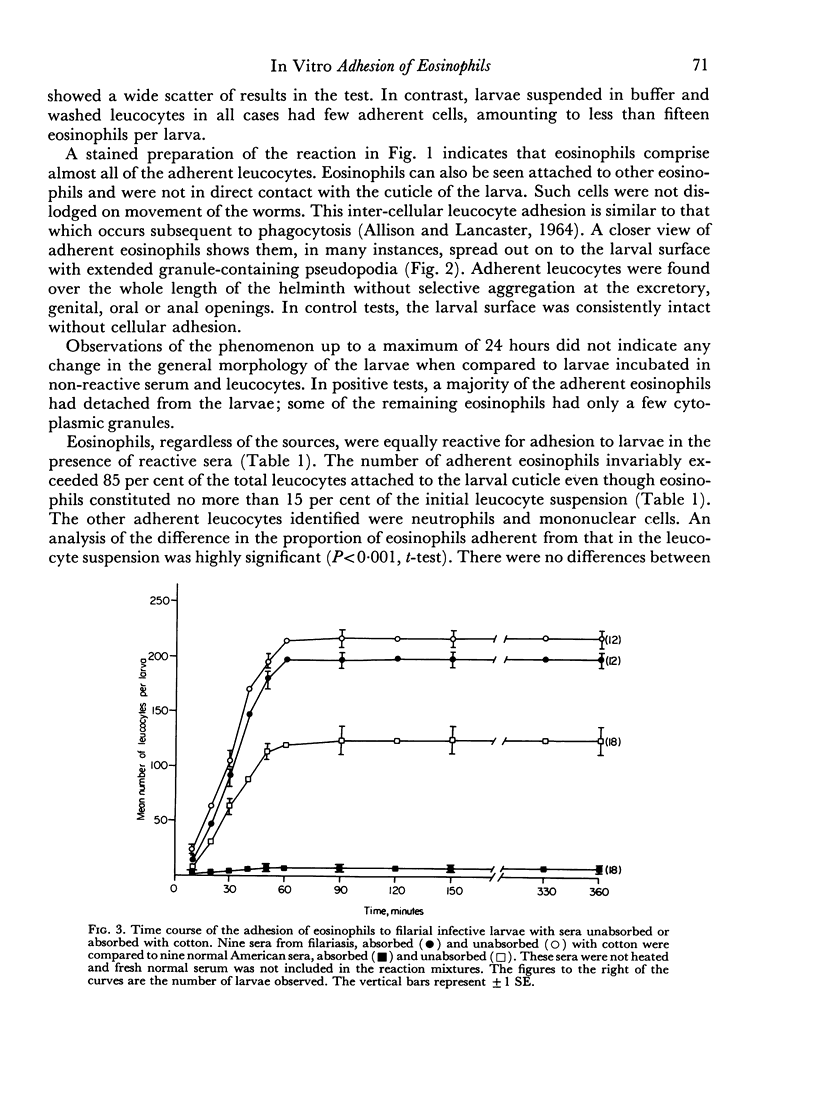
Maximal adhesion of eosinophils was obtained when the larvae were viable and in the presence of immune serum and fresh normal serum during incubation with the leucocytes.
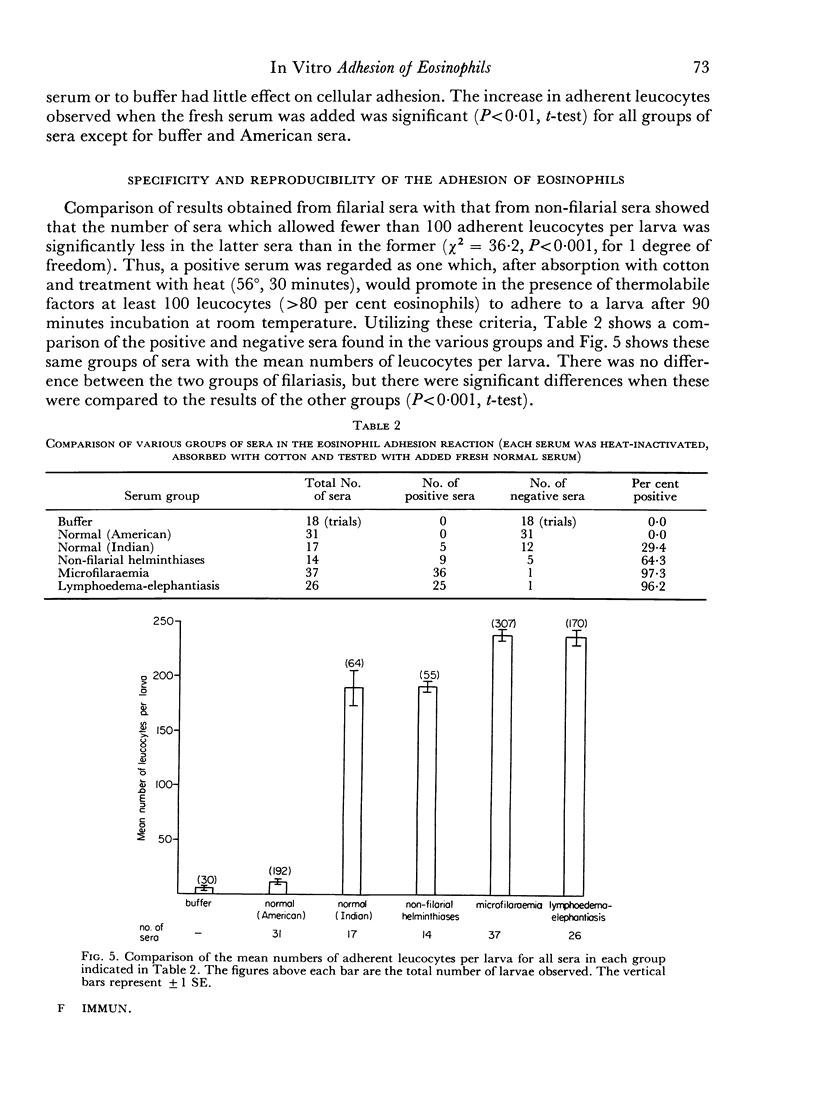
Normal serum was found to induce this adhesion reaction. The responsible factor could be removed by absorption of normal serum with cotton. However, this procedure had no effect on the reactivity of sera from filariasis cases.
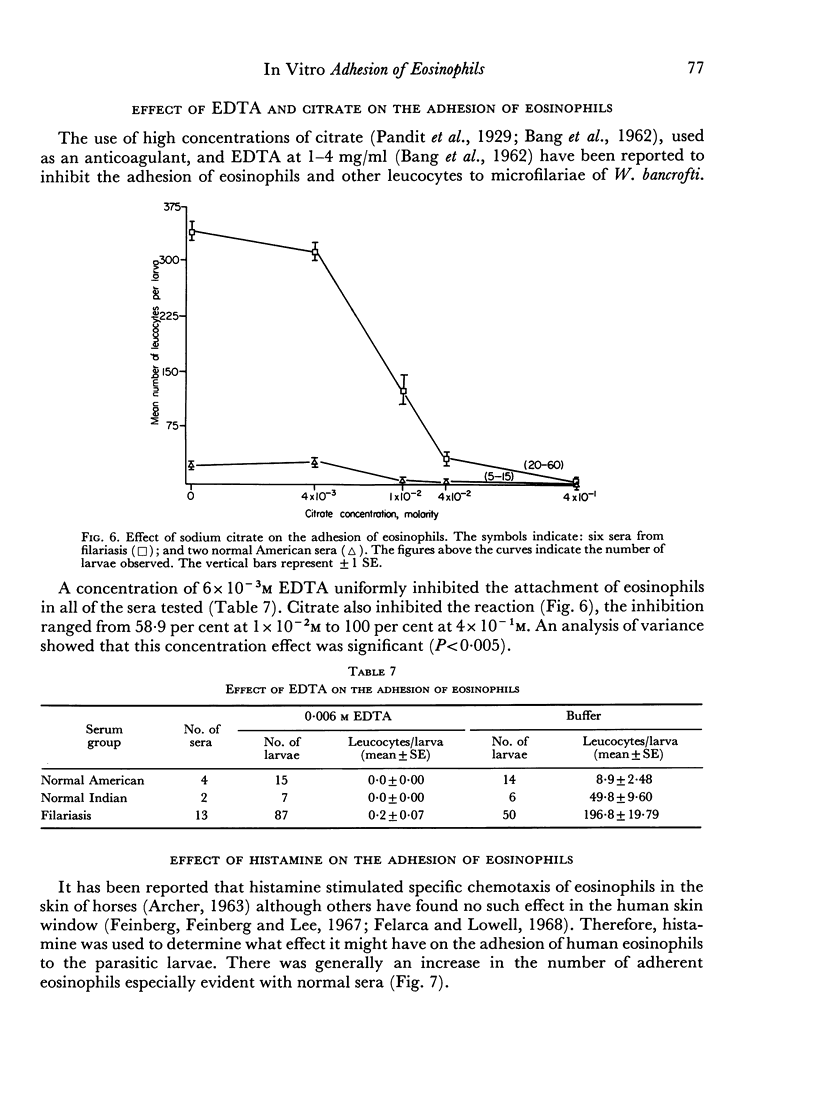
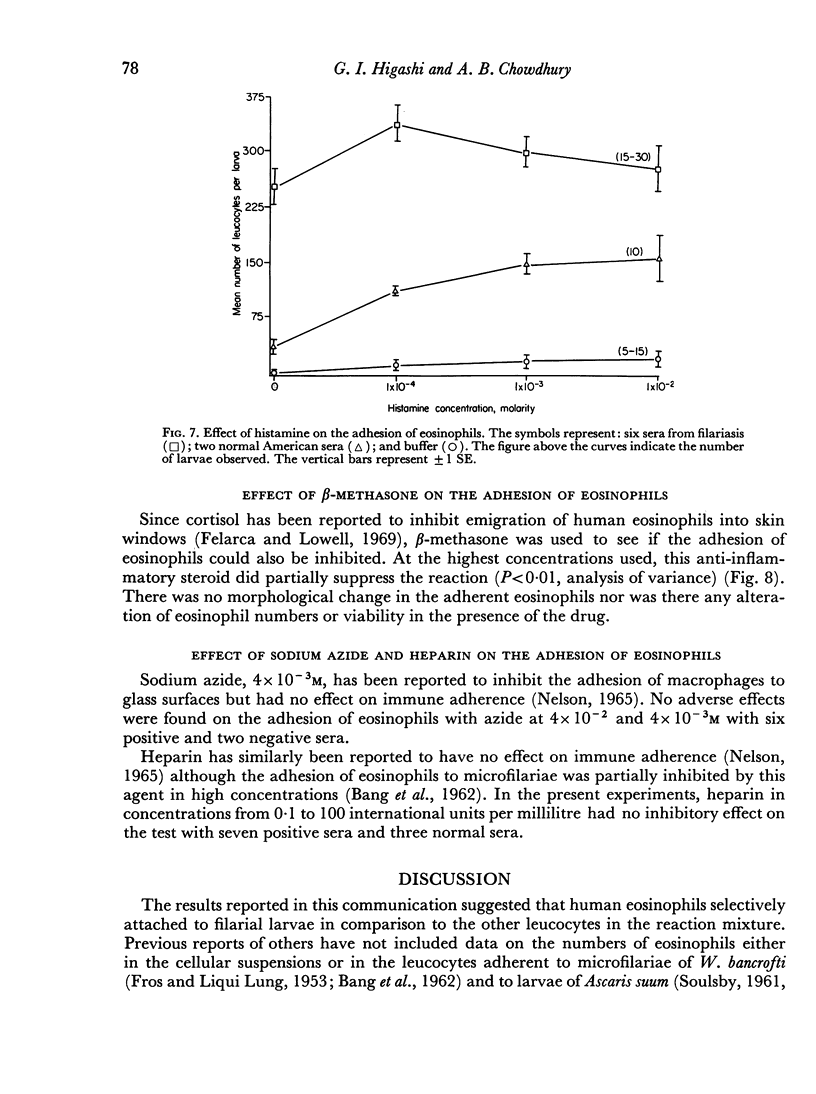
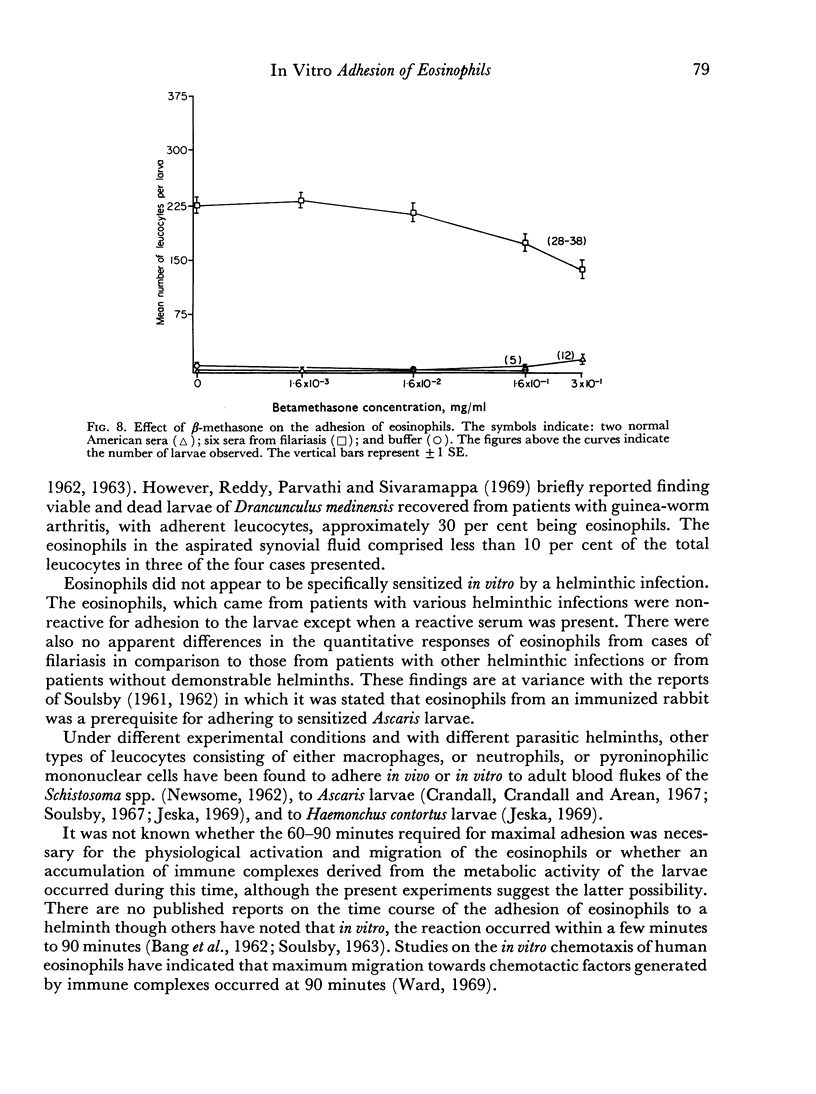
The reaction was almost totally inhibited by EDTA and citrate. The anti-inflammatory steroid, betamethasone, had a moderately inhibitory effect. An unexplained finding was an enhancing effect on the reaction when histamine was added to non-reactive normal serum.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON F., Jr, LANCASTER M. G. STUDIES ON FACTORS WHICH INFLUENCE THE ADHESIVENESS OF LEUKOCYTES IN VITRO. Ann N Y Acad Sci. 1964 Aug 27;116:936–944. doi: 10.1111/j.1749-6632.1964.tb52559.x. [DOI] [PubMed] [Google Scholar]
- BANG F. B., SAHA T. K., BANDYOPADHAYA A. K. Reactions of eosinophils with helminthic larvae in tropical eosinophilia. Bull Calcutta Sch Trop Med. 1962 Oct;10:152–153. [PubMed] [Google Scholar]
- Bryant R. E. Divalent cation requirements for leukocyte adhesiveness. Proc Soc Exp Biol Med. 1969 Mar;130(3):975–977. doi: 10.3181/00379727-130-33701. [DOI] [PubMed] [Google Scholar]
- CHOWDHURY A. B., SCHILLER E. L. Preliminary observations on the application of the fluorescent antibody technique in the laboratory diagnosis of filariasis. Bull Calcutta Sch Trop Med. 1962 Jul;10:97–99. [PubMed] [Google Scholar]
- Cline M. J., Hanifin J., Lehrer R. I. Phagocytosis by human eosinophils. Blood. 1968 Dec;32(6):922–934. [PubMed] [Google Scholar]
- Crandall C. A., Crandall R. B., Arean V. M. Increased resistance in mice to larval Ascaris suum infection induced by Nippostrongylus brasiliensis. J Parasitol. 1967 Feb;53(1):214–215. [PubMed] [Google Scholar]
- FROS J., LIQUI LUNG M. A. Sheathless Microfilaria bancrofti and eosinophilic granulocytes. Doc Med Geogr Trop. 1953 Jun;5(2):116–122. [PubMed] [Google Scholar]
- Felarca A. B., Lowell F. C. Failure to elicit histamine eosinophilotaxis in the skin of atopic man. Description of an improved technique. J Allergy. 1968 Feb;41(2):82–87. doi: 10.1016/0021-8707(68)90016-6. [DOI] [PubMed] [Google Scholar]
- Felarca A. B., Lowell F. C. Local effects of cortisol in the time course of eosinophilotaxis with the use of an improved technique. J Allergy. 1969 Feb;43(2):114–118. doi: 10.1016/0021-8707(69)90131-2. [DOI] [PubMed] [Google Scholar]
- HANKS J. H., WALLACE J. H. Determination of cell viability. Proc Soc Exp Biol Med. 1958 May;98(1):188–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- Henson P. M. The adherence of leucocytes and platelets induced by fixed IgG antibody or complement. Immunology. 1969 Jan;16(1):107–121. [PMC free article] [PubMed] [Google Scholar]
- Jeska E. L. Mouse peritoneal exudate cell reactions to parasitic worms. I. Cell adhesion reactions. Immunology. 1969 Jun;16(6):761–771. [PMC free article] [PubMed] [Google Scholar]
- KETCHEL M. M., FAVOUR C. B. The acceleration and inhibition of migration of human leucocytes in vitro by plasma protein fractions. J Exp Med. 1955 Jun 1;101(6):647–663. doi: 10.1084/jem.101.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWSOME J. Immune opsonins in Schistosoma infestations. Nature. 1962 Sep 22;195:1175–1179. doi: 10.1038/1951175a0. [DOI] [PubMed] [Google Scholar]
- Reddy C. R., Parvathi G., Sivaramappa M. Adhesion of white blood cells to guinea-worm larvae. Am J Trop Med Hyg. 1969 May;18(3):379–381. doi: 10.4269/ajtmh.1969.18.379. [DOI] [PubMed] [Google Scholar]
- Rozeboom L. E., Bhattacharya N. C., Gilotra S. K. Observations on the transmission of filariasis in urban Calcutta. Am J Epidemiol. 1968 May;87(3):616–631. doi: 10.1093/oxfordjournals.aje.a120852. [DOI] [PubMed] [Google Scholar]
- SKARNES R. C., WATSON D. W. Antimicrobial factors of normal tissues and fluids. Bacteriol Rev. 1957 Dec;21(4):273–294. doi: 10.1128/br.21.4.273-294.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOULSBY E. J. Immune mechanisms in helminth infections. Vet Rec. 1961 Oct 28;73:1053–1058. [PubMed] [Google Scholar]
- SOULSBY E. J. THE NATURE AND ORIGIN OF THE FUNCTIONAL ANTIGENS IN HELMINTH INFECTIONS. Ann N Y Acad Sci. 1963 Dec 30;113:492–509. doi: 10.1111/j.1749-6632.1963.tb40686.x. [DOI] [PubMed] [Google Scholar]
- SPEIRS R. S. The principles of eosinophil diluents. Blood. 1952 May;7(5):550–554. [PubMed] [Google Scholar]
- Sadun E. H., Duxbury R. E., Gore R. W., Stechschulte D. J. Homologous passive cutaneous anaphylactic and fluorescent antibody reactions in rabbits infected with Dirofilaria uniformis. J Infect Dis. 1967 Oct;117(4):317–326. doi: 10.1093/infdis/117.4.317. [DOI] [PubMed] [Google Scholar]
- TURK J. L. The immune-adherence activity of normal serum. Br J Exp Pathol. 1959 Apr;40(2):97–106. [PMC free article] [PubMed] [Google Scholar]
- WONG M. M. STUDIES ON MICROFILAREMIA IN DOGS. II. LEVELS OF MICROFILAREMIA IN RELATION TO IMMUNOLOGIC RESPONSES OF THE HOST. Am J Trop Med Hyg. 1964 Jan;13:66–77. [PubMed] [Google Scholar]