Abstract
Ficoll, a polymer of sucrose, has several advantages over proteins for preparation of density gradients used to study mammalian cells. In addition to its ease of preparation, Ficoll as a result of being an uncharged molecule, does not bind ions from solution and, therefore, does not affect the osmolality of the medium in which it is dissolved.
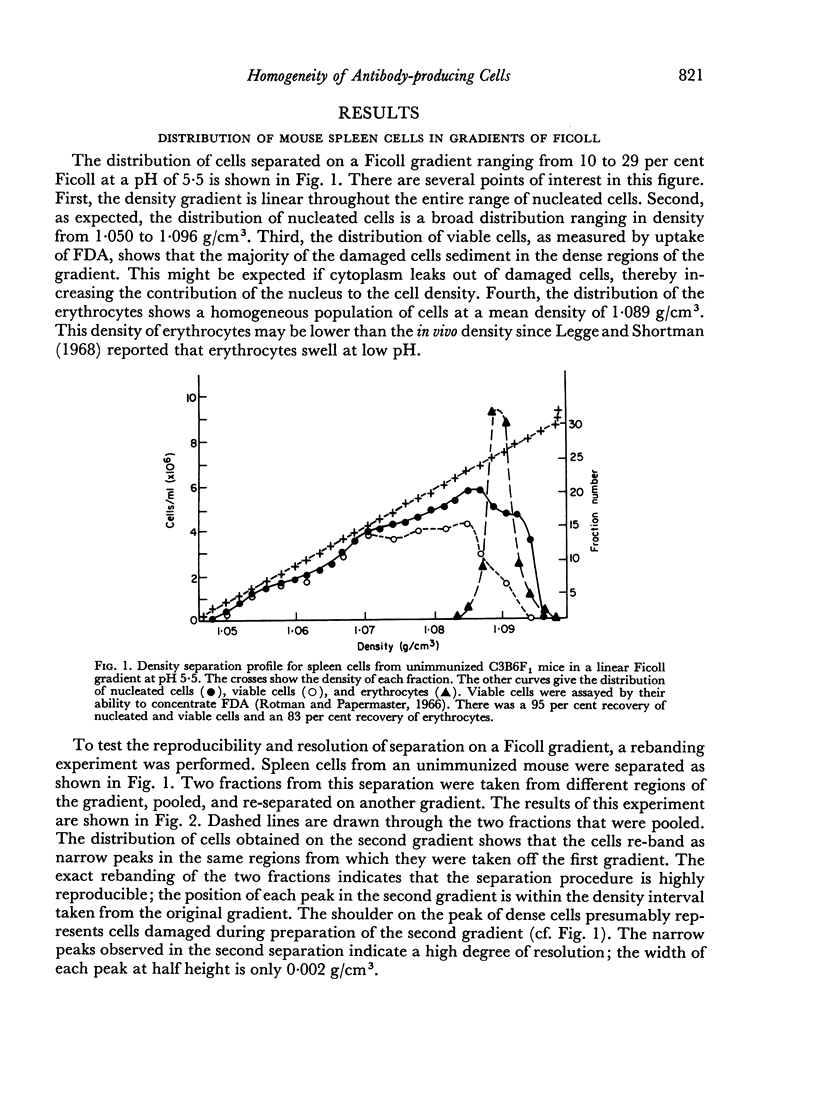
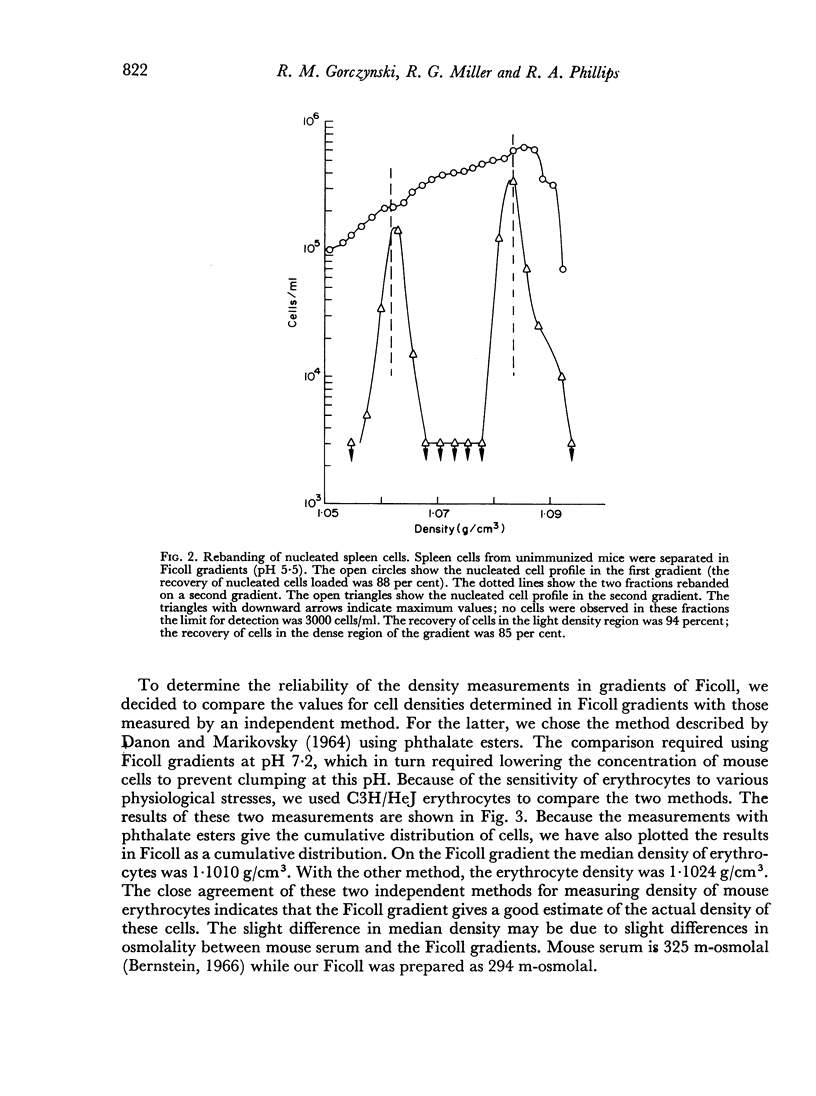
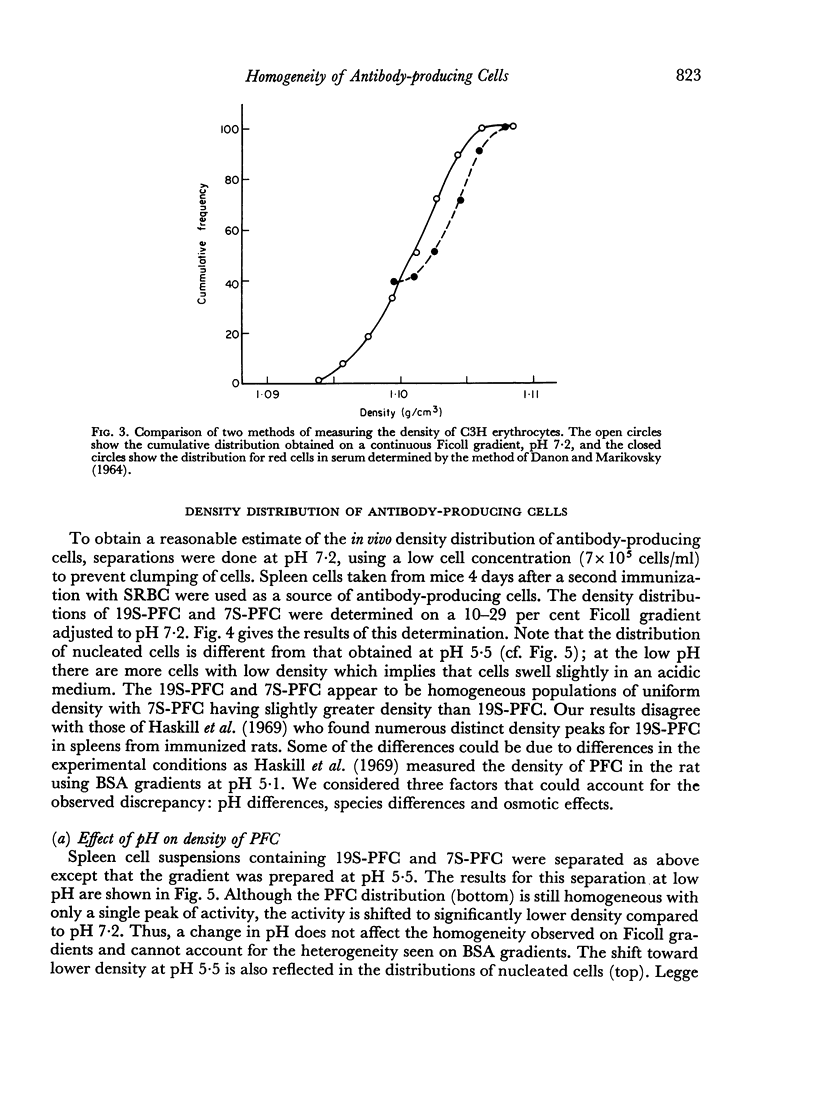
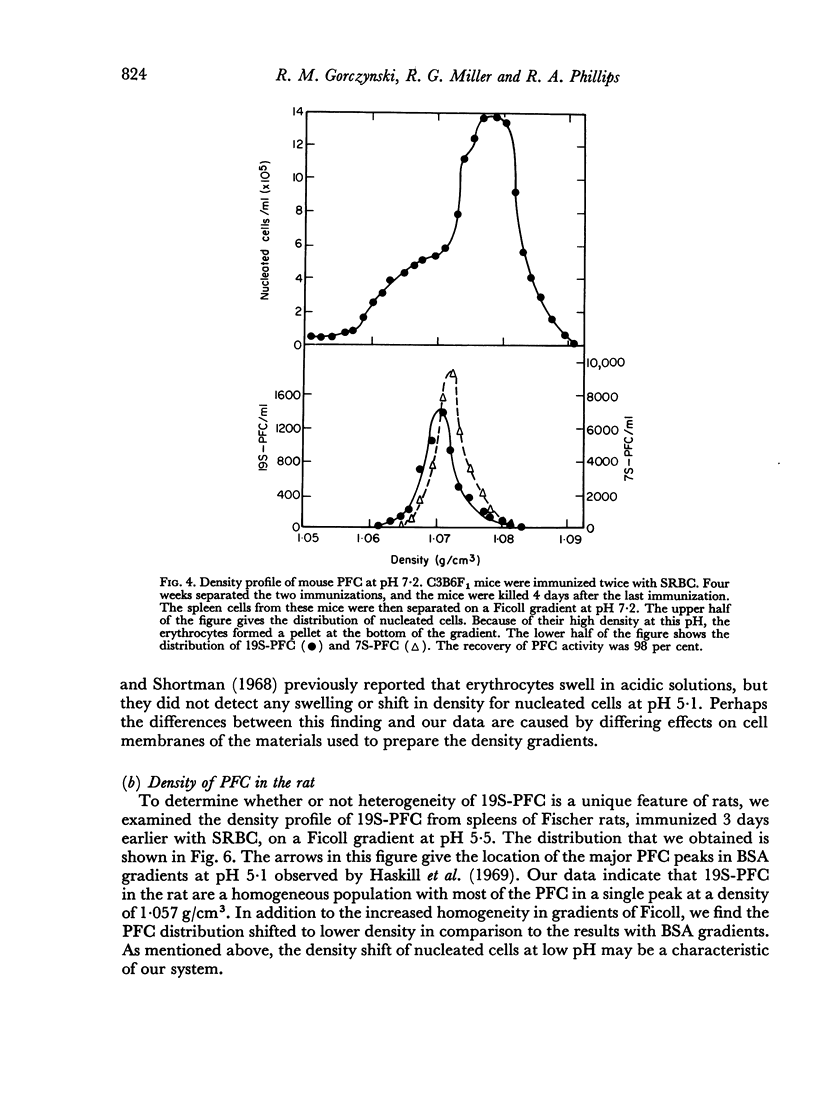
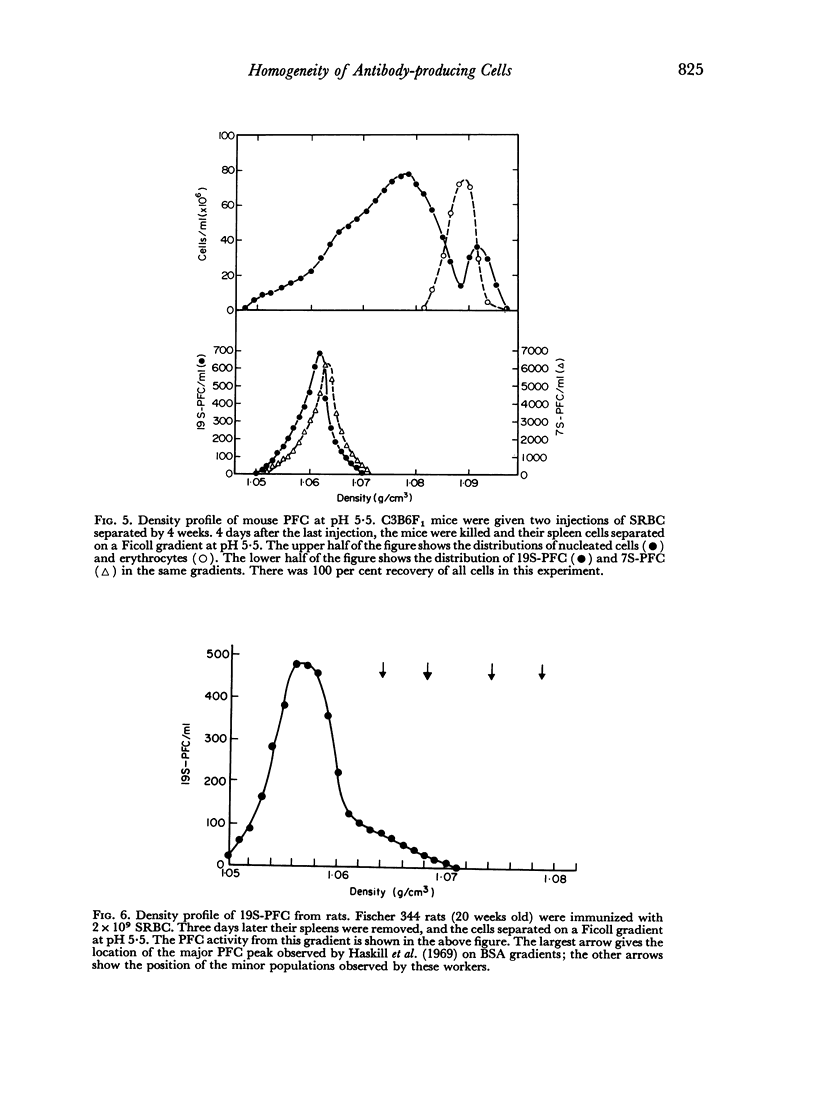
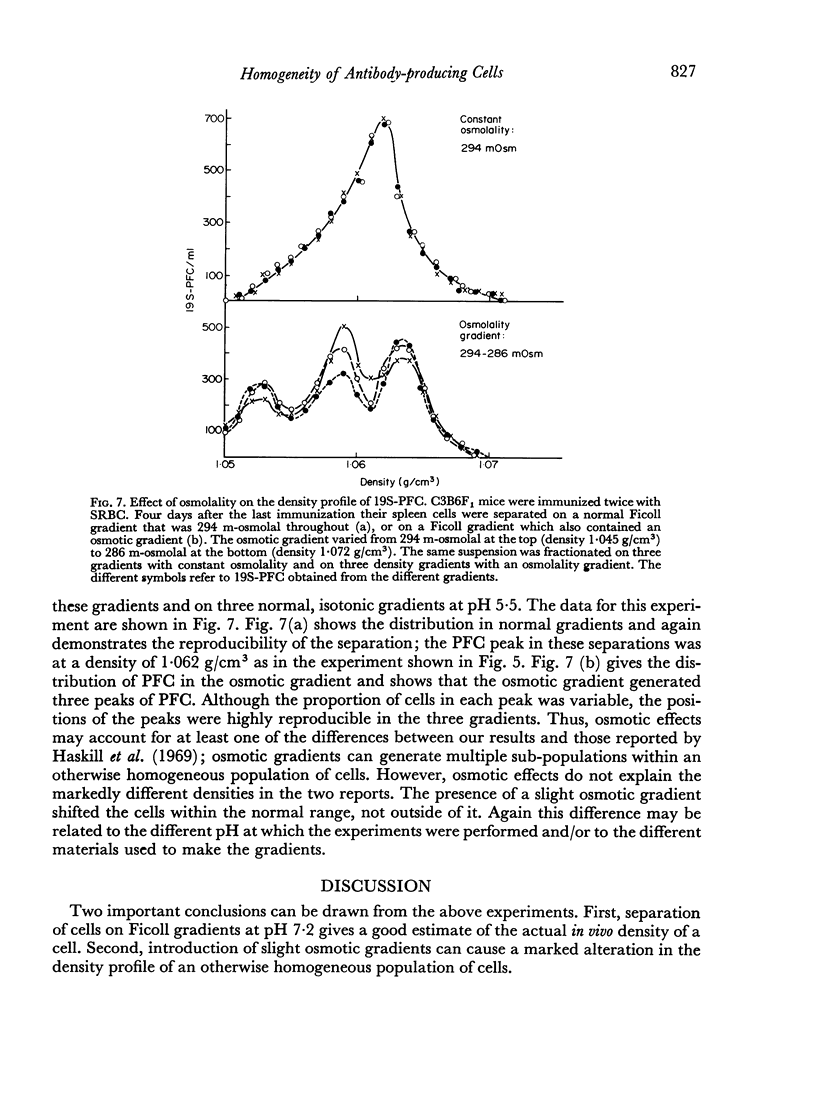
In studies on antibody-producing cells we found that the measurement of buoyant density in Ficoll at pH 7.2 gives an accurate measurement of cell density. In such gradients antibody-producing cells band in a single peak at a density of 1.070 g/cm3. Two factors have a profound influence on the density profile of antibody-producing cells. Lowering the pH to 5.5 lowers their buoyant density to 1.062 g/cm3. Creation of an inverse osmotic gradient varying from 294 m-osmolal at the top of the gradient to 286 m-osmolal at the bottom splits the single peak of antibody-producing cells into three distinct peaks. This effect of osmotic gradients could account for the multiple peaks observed when cells are separated on protein gradients.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cunningham A. J. The morphology of antibody-forming cells in the mouse. Aust J Exp Biol Med Sci. 1968 Apr;46(2):141–153. doi: 10.1038/icb.1968.12. [DOI] [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- Harris T. N., Hummeler K., Harris S. Electron microscopic observations on antibody-producing lymph node cells. J Exp Med. 1966 Jan 1;123(1):161–172. doi: 10.1084/jem.123.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill J. S., Legge D. G., Shortman K. Density distribution analysis of cells forming 19S hemolytic antibody in the rat. J Immunol. 1969 Mar;102(3):703–712. [PubMed] [Google Scholar]
- Legge D. G., Shortman K. The effect of pH on the volume, density and shape of erythrocytes and thymic lymphocytes. Br J Haematol. 1968 Mar;14(3):323–335. doi: 10.1111/j.1365-2141.1968.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Niewisch H., Vogel H., Matioli G. Concentration, quantitation, and identification of hemopoietic stem cells. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2261–2267. doi: 10.1073/pnas.58.6.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K. The separation of different cell classes from lymphoid organs. II. The purification and analysis of lymphocyte populations by equilibrium density gradient centrifugation. Aust J Exp Biol Med Sci. 1968 Aug;46(4):375–396. doi: 10.1038/icb.1968.32. [DOI] [PubMed] [Google Scholar]
- WALDER A. I., LUNSETH J. B. A technic for separation of the cells of the gastric mucosa. Proc Soc Exp Biol Med. 1963 Feb;112:494–496. doi: 10.3181/00379727-112-28086. [DOI] [PubMed] [Google Scholar]
- van Dicke KA Hooft J. I., van Bekkum D. W. The selective elimination of immunologically competent cells from bone marrow and lymphatic cell mixtures. II. Mouse spleen cell fractionation on a discontinuous albumin gradient. Transplantation. 1968 Jul;6(4):562–570. doi: 10.1097/00007890-196807000-00009. [DOI] [PubMed] [Google Scholar]


