Abstract
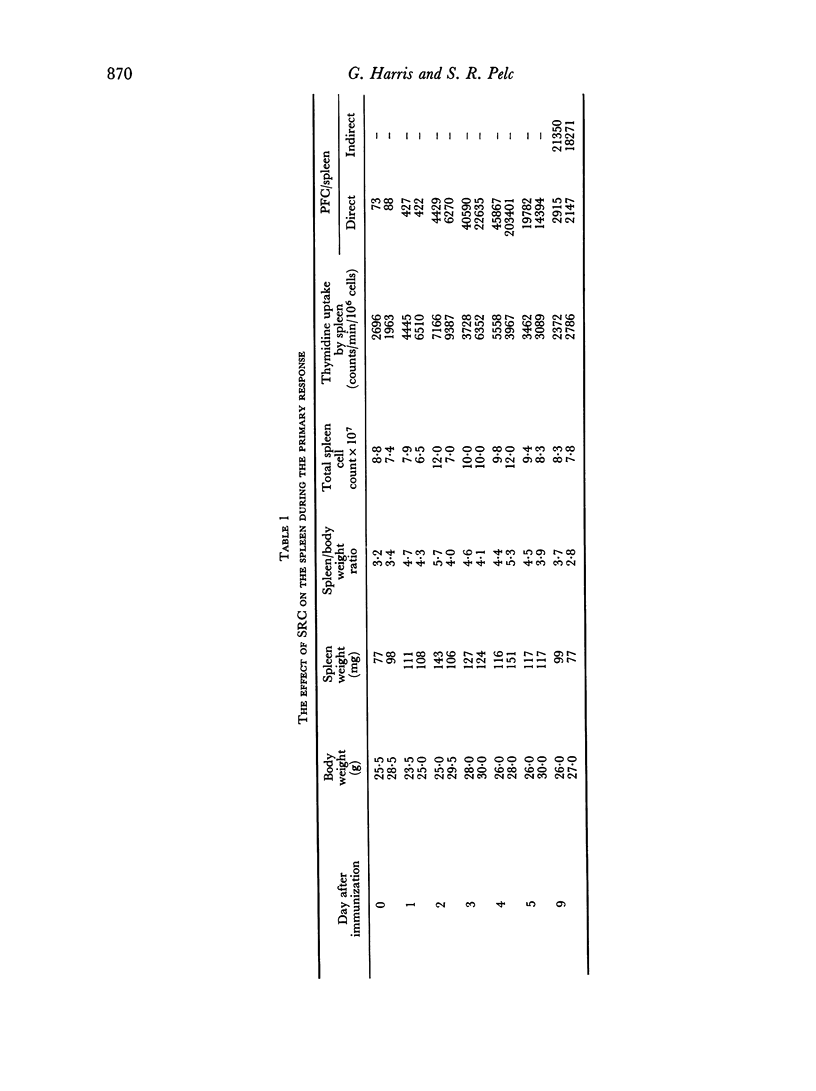
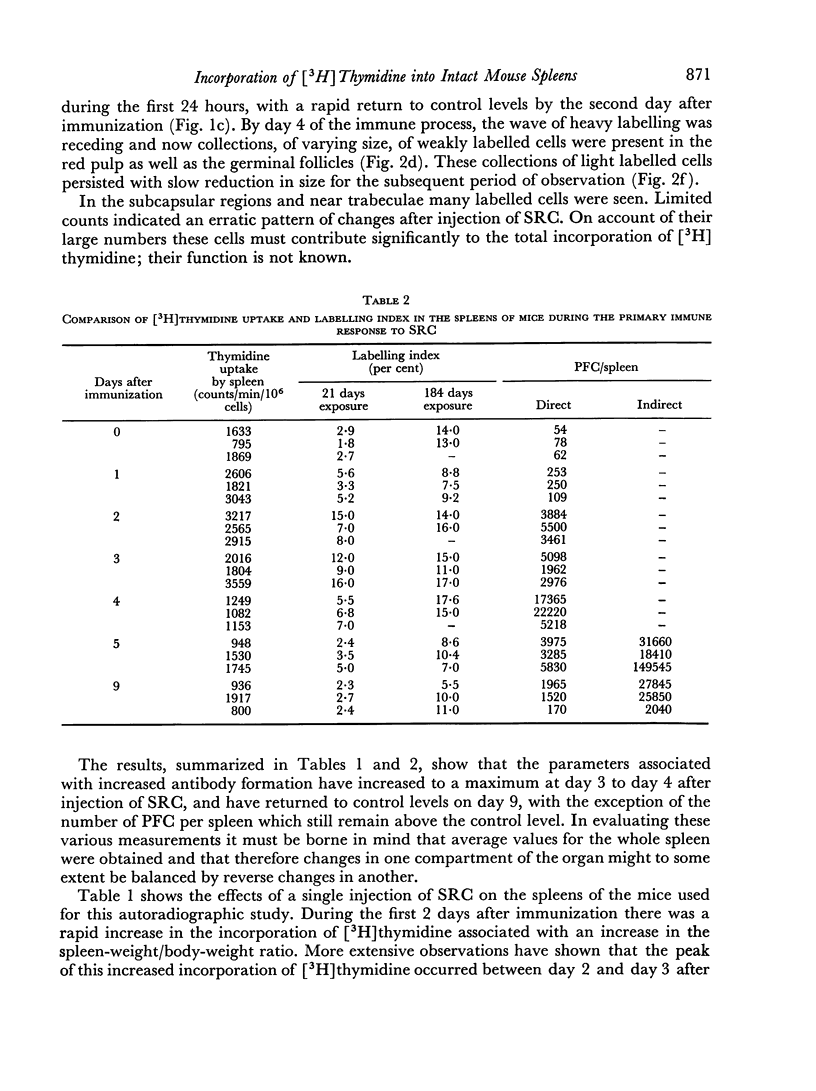
The incorporation of [3H]thymidine, administered shortly before killing, into the spleens of intact mice during the primary immune response to SRC has been studied, using autoradiography with exposure periods of 184 days before development.
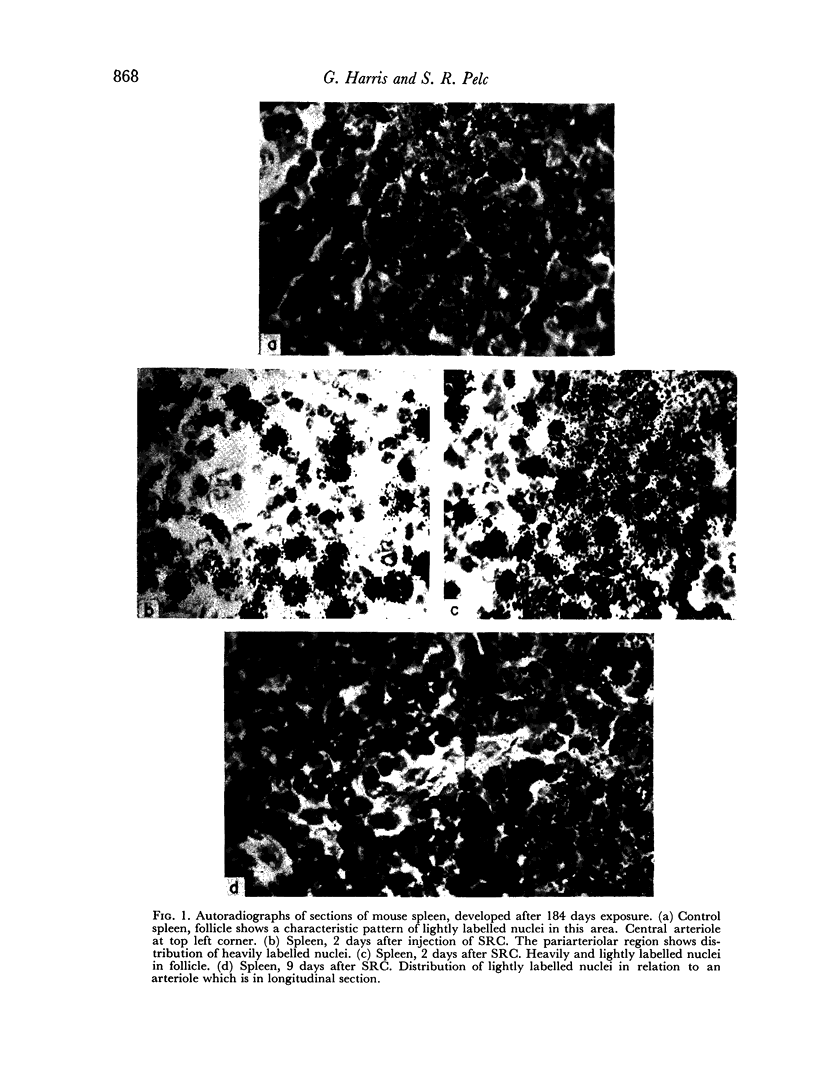
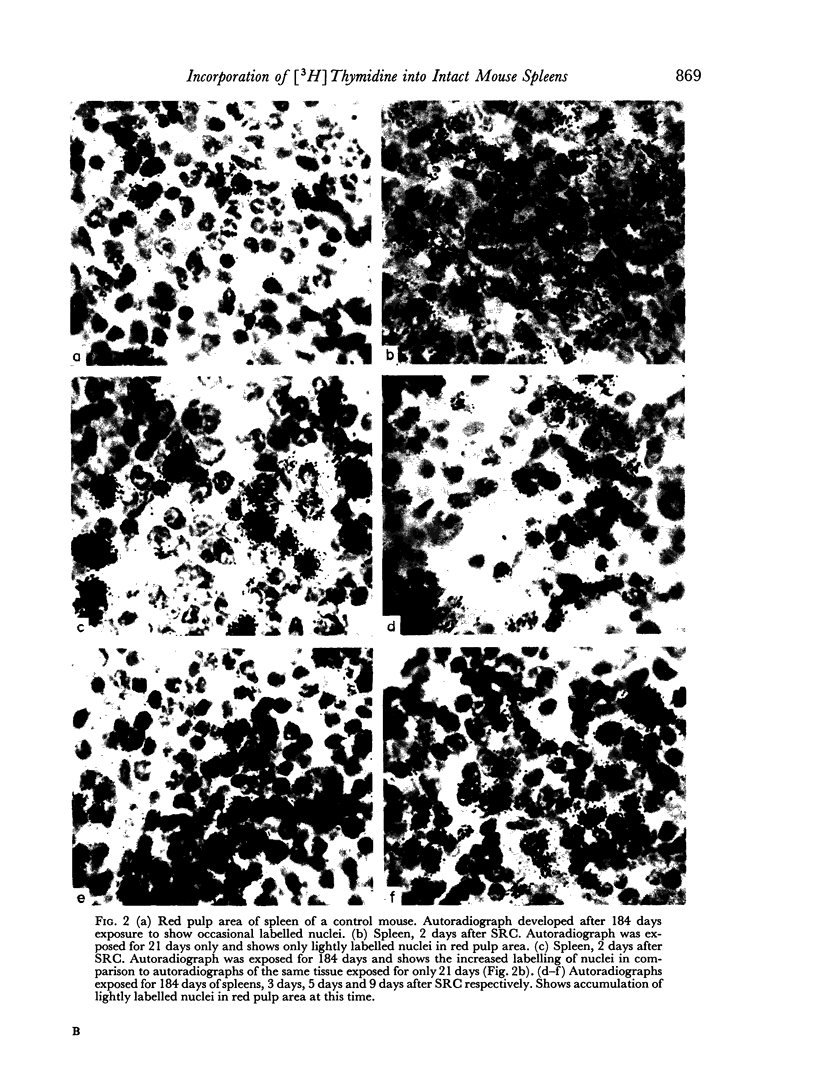
A mixture of weakly and heavily labelled nuclei were situated in well-defined areas of the follicles. A marked increase of heavily labelled nuclei coincided with an increased uptake of [3H]thymidine into the spleen DNA during the first 3 days of the immune response. At this time the number of lightly labelled nuclei in the follicles was reduced. The heavily labelled nuclei were first apparent in the periarteriolar zone, then in germinal centres spreading out into the red pulp. After the peak of [3H]thymidine incorporation was over (day 3–4) weakly-labelled nuclei accumulated in the red pulp and persisted until day 9 after the injection of SRC.
It was concluded that many non-dividing cells in mouse spleen were incorporating small amounts of [3H]thymidine into their nuclear DNA. In view of the accumulation of such cells in the red pulp during the course of the immune response to SRC, it was considered that this evidence of DNA synthesis was a manifestation of metabolic turnover of this molecule and relevant to the immune process. From the data presented it was also concluded that only a small proportion of the total spleen population engaged in DNA synthesis and proliferation were actually induced to produce specific antibodies. This preliminary investigation showed the complex nature of the immune process leading to antibody synthesis, which requires much further detailed study.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton T. C., Pelc S. R., Tarbit M. H. Formation and loss of DNA in intestinal epithelium. J Cell Sci. 1969 Jul;5(1):45–55. doi: 10.1242/jcs.5.1.45. [DOI] [PubMed] [Google Scholar]
- Dutton R. W., Mishell R. I. Cell populations and cell proliferation in the in vitro response of normal mouse spleen to heterologous erythrocytes. Analysis by the hot pulse technique. J Exp Med. 1967 Sep 1;126(3):443–454. doi: 10.1084/jem.126.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANNA M. G., Jr AN AUTORADIOGRAPHIC STUDY OF THE GERMINAL CENTER IN SPLEEN WHITE PULP DURING EARLY INTERVALS OF THE IMMUNE RESPONSE. Lab Invest. 1964 Feb;13:95–104. [PubMed] [Google Scholar]
- Harris G. Antibody production in vitro. I. Single cell studies of the secondary response to sheep erythrocytes. J Exp Med. 1968 Apr 1;127(4):661–674. doi: 10.1084/jem.127.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G., Littleton R. J. The effects of antigens and of phytohemagglutinin on rabbit spleen cell suspensions. J Exp Med. 1966 Oct 1;124(4):621–634. doi: 10.1084/jem.124.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELC S. R. Incorporation of tritiated thymidine in various organs of the mouse. Nature. 1962 Feb 24;193:793–795. doi: 10.1038/193793a0. [DOI] [PubMed] [Google Scholar]
- PELC S. R. LABELLING OF DNA AND CELL DIVISION IN SO CALLED NON-DIVIDING TISSUES. J Cell Biol. 1964 Jul;22:21–28. doi: 10.1083/jcb.22.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELC S. R. Nuclear uptake of labelled adenine in the seminal vesicle of the mouse. Exp Cell Res. 1958 Apr;14(2):301–315. doi: 10.1016/0014-4827(58)90188-5. [DOI] [PubMed] [Google Scholar]
- POTTER R. L., NYGAARD O. F. The conversion of thymidine to thymine nucleotides and deoxyribonucleic acid in vivo. J Biol Chem. 1963 Jun;238:2150–2155. [PubMed] [Google Scholar]
- Pelc S. R. Incorporation of [3H]thymidine in mouse spleen. J Cell Sci. 1968 Jun;3(2):263–272. doi: 10.1242/jcs.3.2.263. [DOI] [PubMed] [Google Scholar]
- Pelc S. R. Turnover of DNA and function. Nature. 1968 Jul 13;219(5150):162–163. doi: 10.1038/219162a0. [DOI] [PubMed] [Google Scholar]
- Pelc S. R., Viola-Magni M. P. 3. Decrease of labeled DNA in cells of the adrenal medulla after intermittent exposure to cold. J Cell Biol. 1969 Aug;42(2):460–468. doi: 10.1083/jcb.42.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. A., Fitch F. W., Mosier D. E., Solliday S., Coppleson L. W., Brown B. W. The rate of division of antibody-forming cells during the early primary immune response. J Exp Med. 1968 May 1;127(5):983–1002. doi: 10.1084/jem.127.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O. Antibody variability. Somatic recombination between the elements of "antibody gene pairs" may explain antibody variability. Science. 1967 Jul 21;157(3786):267–273. doi: 10.1126/science.157.3786.267. [DOI] [PubMed] [Google Scholar]
- Tannenberg W. J., Malaviya A. N. The life cycle of antibody-forming cells. I. The generation time of 19S hemolytic plaque-forming cells during the primary and secondary responses. J Exp Med. 1968 Nov 1;128(5):895–925. doi: 10.1084/jem.128.5.895. [DOI] [PMC free article] [PubMed] [Google Scholar]




