Abstract
Because of the failure of chicken antibody to activate the first component (C1) of guinea-pig complement a haemolytic system for the measurement of chicken C1 was developed. Chicken antisera to influenza and Rous sarcoma viruses were then assayed by the measurement of the fixation of chicken C1. Antibody titres obtained with this C1-fixation assay correlated well with those obtained by other methods.
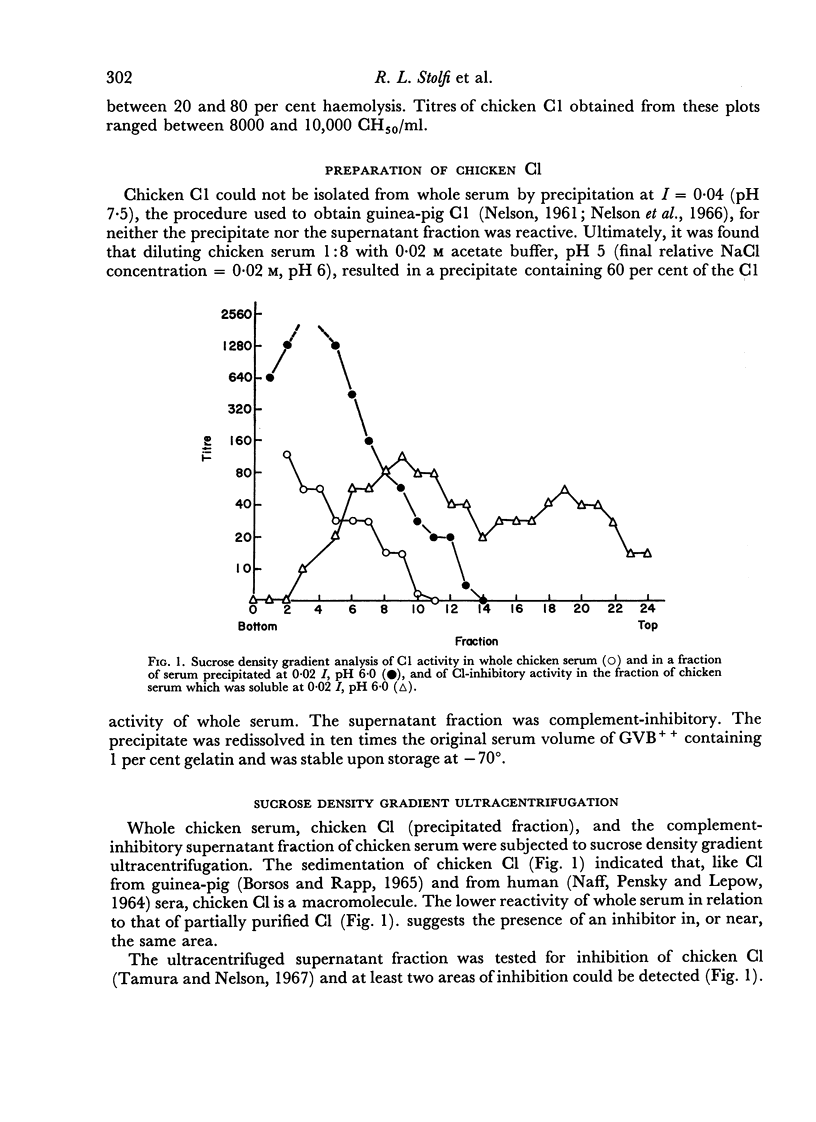
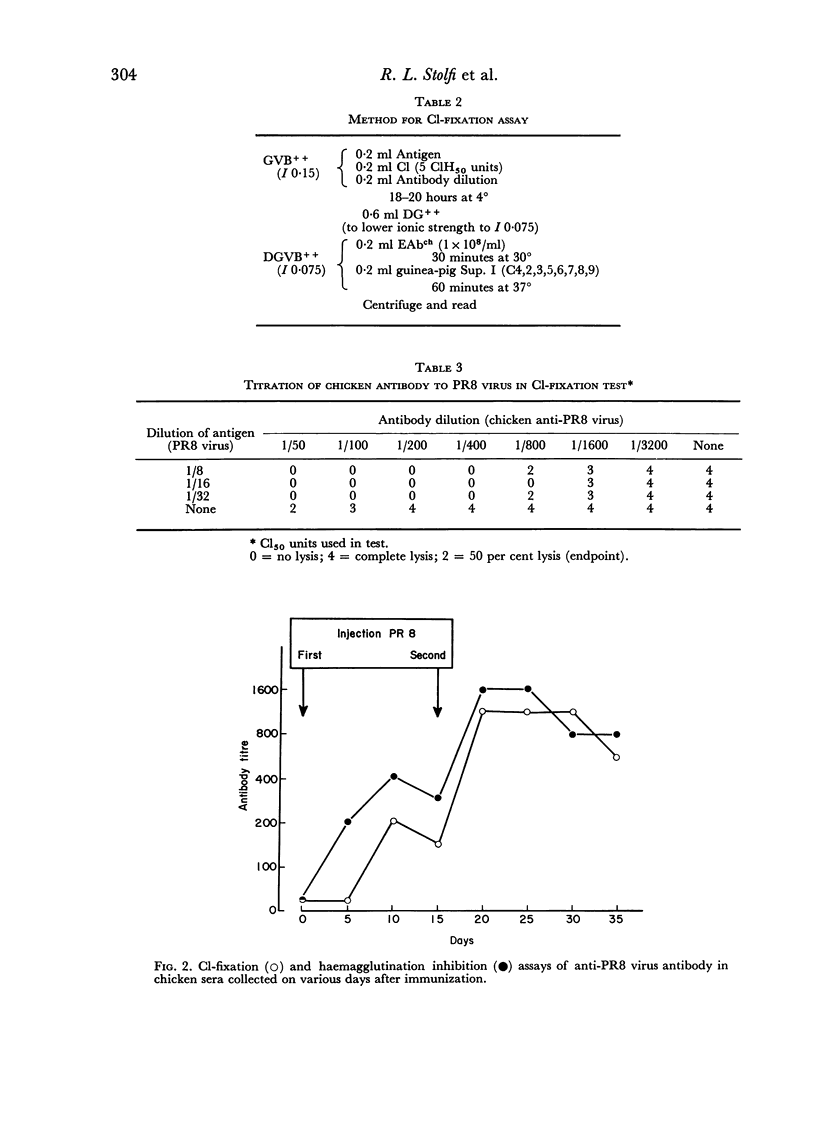
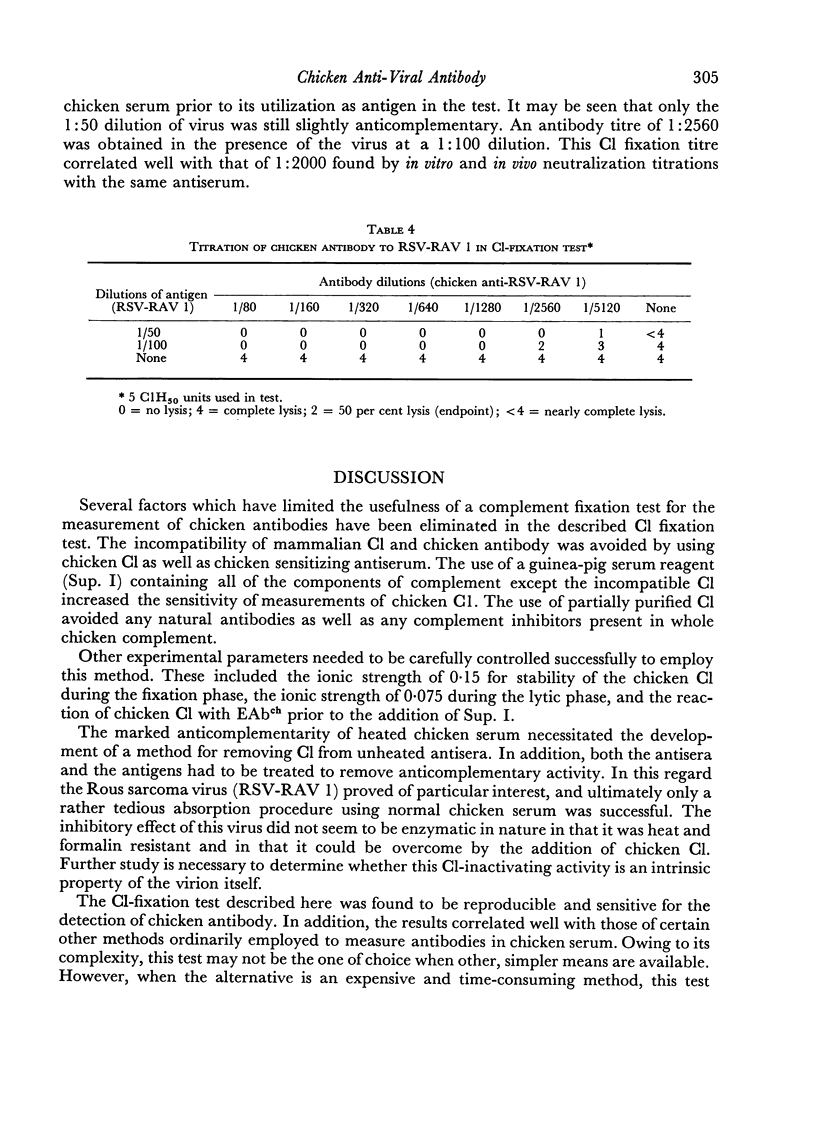
The haemolytic system consisted of sheep erythrocytes sensitized with chicken antibody, partially purified preparations of chicken C1, and a guinea-pig serum reagent free of C1, but containing the remaining eight components of complement. Measurement of the residual chicken C1 by this method was profoundly influenced by ionic strength during both the fixation and the lytic phases, and by the order of addition of the reagents.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENSON H. N., BRUMFIELD H. P., POMEROY B. S. Requirement of avian C'1 for fixation of guinea pig complement by avian antibody-antigen complexes. J Immunol. 1961 Nov;87:616–622. [PubMed] [Google Scholar]
- BORSOS T., RAPP H. J. ESTIMATION OF MOLECULAR SIZE OF COMPLEMENT COMPONENTS BY SEPHADEX CHROMATOGRAPHY. J Immunol. 1965 Apr;94:510–513. [PubMed] [Google Scholar]
- Lefkowitz S. S., Williams J. A., Howard B. E., Sigel M. M. Adenovirus antibody measured by the passive hemagglutination test. J Bacteriol. 1966 Jan;91(1):205–212. doi: 10.1128/jb.91.1.205-212.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAFF G. B., PENSKY J., LEPOW I. H. THE MACROMOLECULAR NATURE OF THE FIRST COMPONENT OF HUMAN COMPLEMENT. J Exp Med. 1964 Apr 1;119:593–613. doi: 10.1084/jem.119.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- OKAZAKI W., PURCHASE H. G., FREDRICKSON T. N., BURMESTER B. R. Modification of complement fixation test for estimation of Rous sarcoma virus antibodies in turkey and chicken serums. Proc Soc Exp Biol Med. 1962 Nov;111:377–380. doi: 10.3181/00379727-111-27797. [DOI] [PubMed] [Google Scholar]
- ORLANS E., ROSE M. E., CLAPP K. H. Fowl antibody: V. The interactions of fresh and heated fowl serum and of guinea-pig complement measured by the lysis of sensitized red cells. Immunology. 1962 Nov;5:649–655. [PMC free article] [PubMed] [Google Scholar]
- ROSE M. E., ORLANS E. Fowl antibody: III. Its haemolytic activity with complements of various species and some properties of fowl complement. Immunology. 1962 Nov;5:633–641. [PMC free article] [PubMed] [Google Scholar]
- ROSE M. E., ORLANS E. Fowl antibody: IV. The estimation of haemolytic fowl complement. Immunology. 1962 Nov;5:642–648. [PMC free article] [PubMed] [Google Scholar]
- RUBIN H., CORNELIUS A., FANSHIER L. The pattern of congenital transmission of an avian lekosis virus. Proc Natl Acad Sci U S A. 1961 Jul 15;47:1058–1069. doi: 10.1073/pnas.47.7.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. E. Avian Complement-Fixation Tests. Can J Comp Med Vet Sci. 1947 Aug;11(8):236–245. [PMC free article] [PubMed] [Google Scholar]
- Tamura N., Nelson R. A., Jr Three naturally-occurring inhibitors of components of complement in guinea pig and rabbit serum. J Immunol. 1967 Sep;99(3):582–589. [PubMed] [Google Scholar]


