Abstract
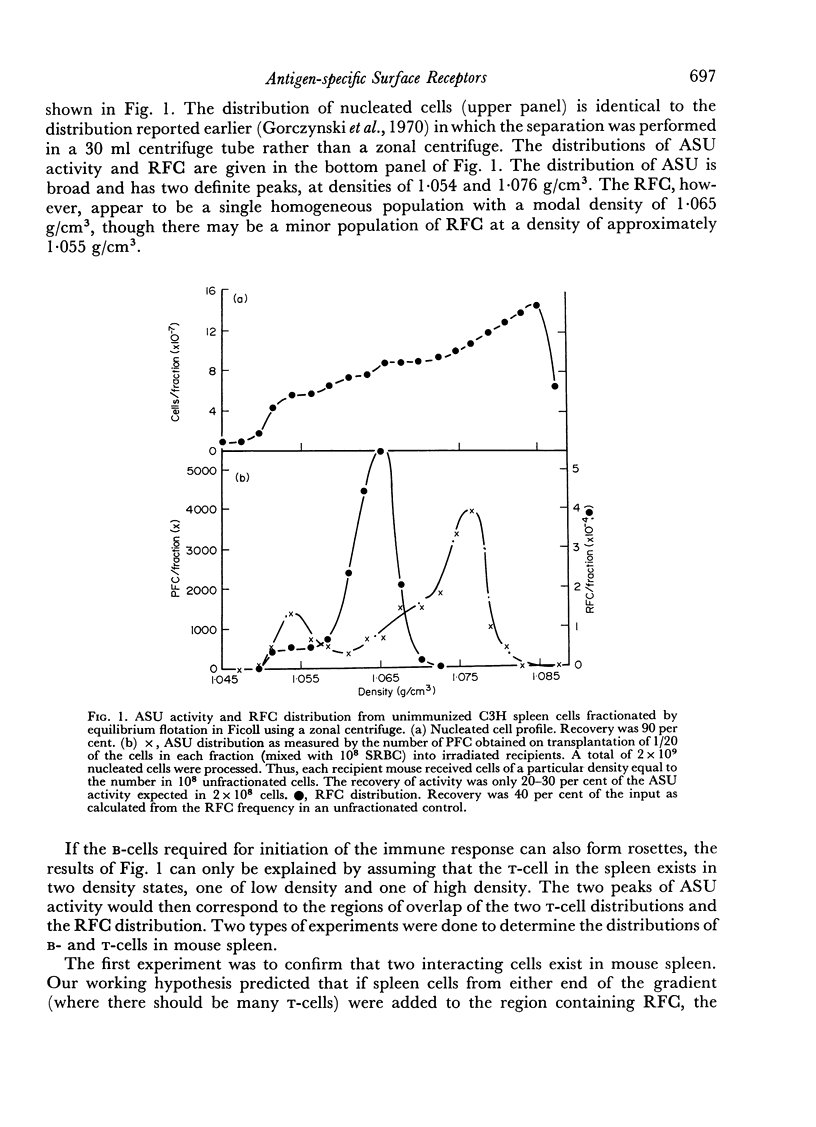
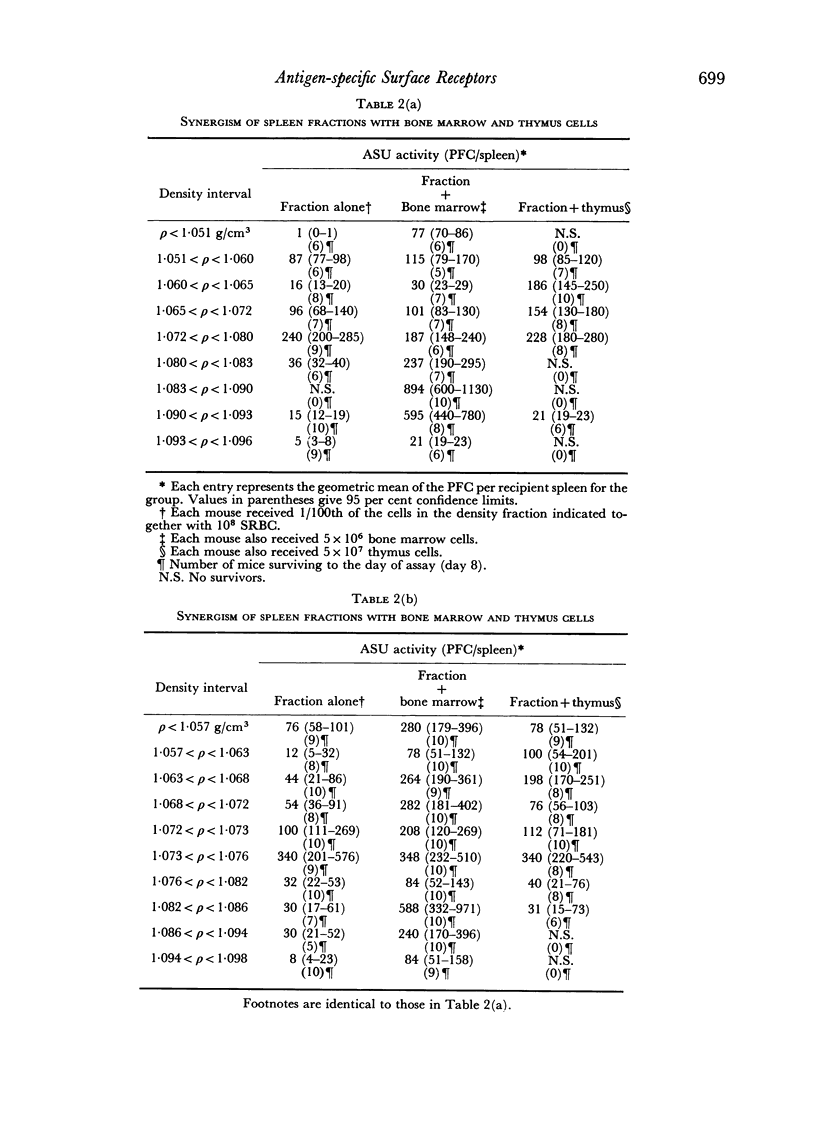
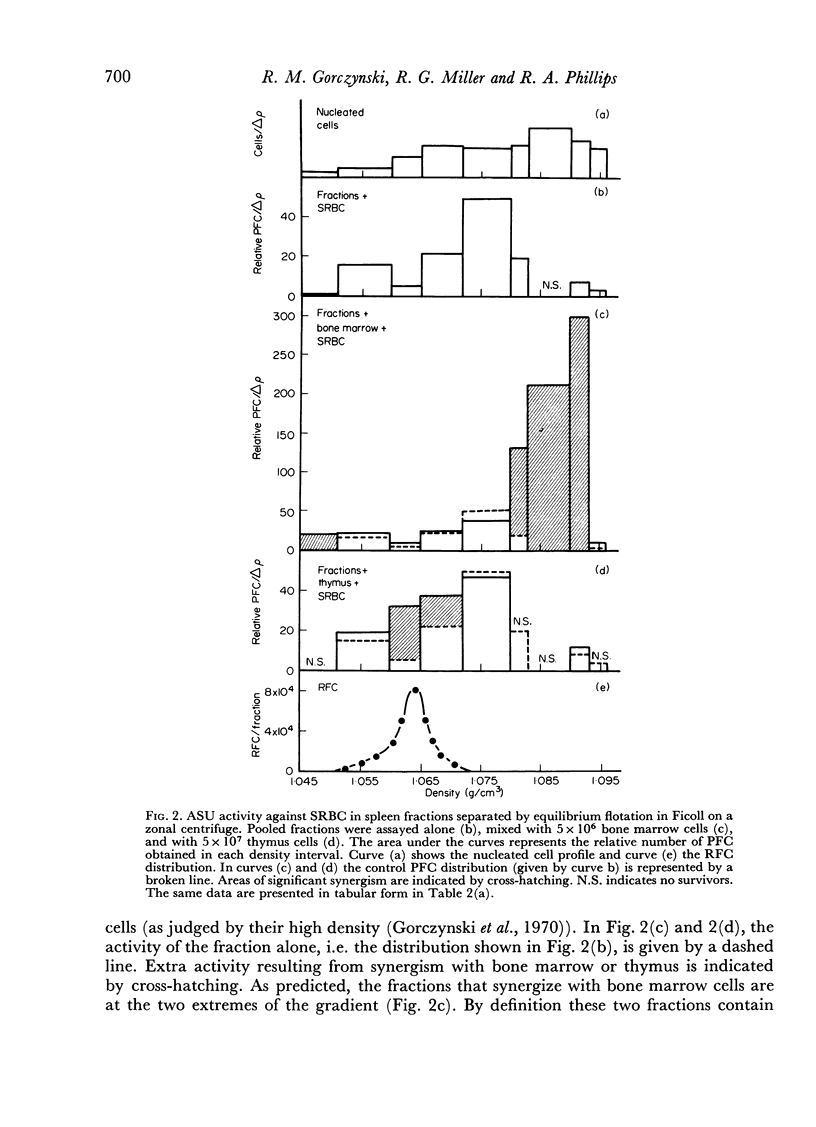
Initiation of an immune response to sheep erythrocytes by the transplantation of spleen cells into irradiated recipients is thought to involve an interaction between two functionally different lymphoid cells. If such populations actually exist, it should be possible to separate them from each other and to study the properties of each type of cell. To this end, mouse spleen cells have been fractionated using equilibrium centrifugation in density gradients of Ficoll. Two functionally different populations of spleen cells were obtained. Neither population was active in initiating an immune response by itself on transplantation into irradiated mice, but mixtures of the two populations were. One population of spleen cells was also active when transplanted together with normal mouse bone marrow and the other population was active when mixed with thymus cells from normal mice. These results indicate that two different spleen cells must interact to initiate an immune response in vivo.
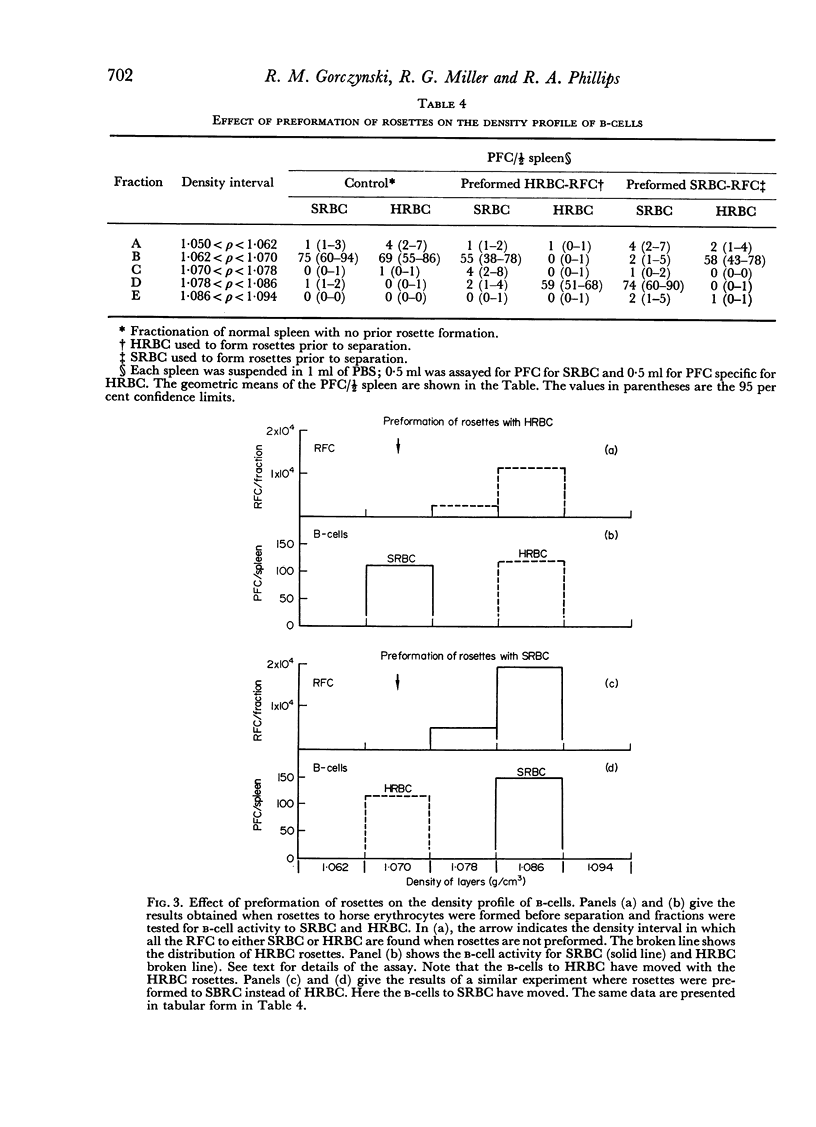
The specificity of the cell that synergizes with thymus cells was investigated further and shown to have antigen-specific receptors on its surface. These cells can be recognized by their ability to make rosettes with erythrocyte antigens. Thus, the `background' rosette-forming cells found in unimmunized mice are actually progenitors of antibody-producing cells.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Byrt P. Specific inactivation of antigen-reactive cells with 125I-labelled antigen. Nature. 1969 Jun 28;222(5200):1291–1292. doi: 10.1038/2221291a0. [DOI] [PubMed] [Google Scholar]
- Adler F. L., Fishman M., Dray S. Antibody formation initiated in vitro. 3. Antibody formation and allotypic specificity directed by ribonucleic acid from peritoneal exudate cells. J Immunol. 1966 Oct;97(4):554–558. [PubMed] [Google Scholar]
- Bretscher P. A., Cohn M. Minimal model for the mechanism of antibody induction and paralysis by antigen. Nature. 1968 Nov 2;220(5166):444–448. doi: 10.1038/220444a0. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A. Immunologic complementation between thymus and marrow cells--a model for the two-cell theory of immunocompetence. Transplant Rev. 1969;1:92–113. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J. Studies on the cellular basis of IgM immunological memory. The induction of antibody formation in bone marrow cells by primed spleen cells. Immunology. 1969 Dec;17(6):933–942. [PMC free article] [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C., Wolstencroft R. A., Panayi G. S., Matthew M., Morley J., Howson W. T. "Lymphokines": non-antibody mediators of cellular immunity generated by lymphocyte activation. Nature. 1969 Oct 4;224(5214):38–42. doi: 10.1038/224038a0. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M., Miller R. G., Phillips R. A. Homogeneity of antibody-producing cells as analysed by their buoyant density in gradients of Ficoll. Immunology. 1970 Nov;19(5):817–829. [PMC free article] [PubMed] [Google Scholar]
- Gregory C. J., Lajtha L. G. Kinetic study of the production of antibody-forming cells from their precursors. Nature. 1968 Jun 15;218(5146):1079–1081. doi: 10.1038/2181079a0. [DOI] [PubMed] [Google Scholar]
- Groves D. L., Lever W. E., Makinodan T. A model for the interaction of cell types in the generation of hemolytic plaque-forming cells. J Immunol. 1970 Jan;104(1):148–165. [PubMed] [Google Scholar]
- Haskill J. S., Byrt P., Marbrook J. In vitro and in vivo studies of the immune response to sheep erythrocytes using partially purified cell preparations. J Exp Med. 1970 Jan 1;131(1):57–76. doi: 10.1084/jem.131.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. B., L'age-Stehr J., Herzenberg L. A. Immunological memory in mice. II. Cell interactions in the secondary immune response studies by means of immunoglobulin allotype markers. J Exp Med. 1970 Jun 1;131(6):1109–1120. doi: 10.1084/jem.131.6.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. C., Treadwell P. E., Lennox E. S. Antigen-specific synergism in the immune response of irradiated mice given marrow cells and peritoneal cavity cells or extracts. J Exp Med. 1970 Aug 1;132(2):353–367. doi: 10.1084/jem.132.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. V. Target cells for tolerance induction. J Exp Med. 1970 Apr 1;131(4):675–699. doi: 10.1084/jem.131.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Sedimentation analysis of the cells in mice required to initiate an in vivo immune response to sheep erythrocytes. Proc Soc Exp Biol Med. 1970 Oct;135(1):63–67. doi: 10.3181/00379727-135-34988. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The immunogenic capacity of antigen taken up by peritoneal exudate cells. Immunology. 1969 Jan;16(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- Moav N., Harris T. N. Collection of rosette-forming spleen cells by BPA density gradient centrifugation. Proc Soc Exp Biol Med. 1968 Jun;128(2):407–411. doi: 10.3181/00379727-128-33024. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Fitch F. W., Rowley D. A., Davies A. J. Cellular deficit in thymectomized mice. Nature. 1970 Jan 17;225(5229):276–277. doi: 10.1038/225276a0. [DOI] [PubMed] [Google Scholar]
- Munro A., Hunter P. In vitro reconstitution of the immune response of thymus-deprived mice to sheep red blood cells. Nature. 1970 Jan 17;225(5229):277–278. doi: 10.1038/225277a0. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Cunningham A., Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. 3. Chromosomal marker analysis of single antibody-forming cells in reconstituted, irradiated, or thymectomized mice. J Exp Med. 1968 Oct 1;128(4):839–853. doi: 10.1084/jem.128.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osoba D. Some physical and radiobiological properties of immunologically reactive mouse spleen cells. J Exp Med. 1970 Aug 1;132(2):368–383. doi: 10.1084/jem.132.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. A., Miller R. G. Antibody-producing cells: analysis and purification by velocity sedimentation. Cell Tissue Kinet. 1970 Jul;3(3):263–274. doi: 10.1111/j.1365-2184.1970.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Roseman J. X-ray resistant cell required for the induction of in vitro antibody formation. Science. 1969 Sep 12;165(3898):1125–1127. doi: 10.1126/science.165.3898.1125. [DOI] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Cudkowicz G., Priore R. L. Cellular differentiation of the immune system of mice. II. Frequency of unipotent splenic antigen-sensitive units after immunization with sheep erythrocytes. J Exp Med. 1969 Jan 1;129(1):185–199. doi: 10.1084/jem.129.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Diener E., Russell P., Armstrong W. D. The role of nonlymphoid accessory cells in the immune response to different antigens. J Exp Med. 1970 Mar 1;131(3):461–482. doi: 10.1084/jem.131.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K. The separation of different cell classes from lymphoid organs. II. The purification and analysis of lymphocyte populations by equilibrium density gradient centrifugation. Aust J Exp Biol Med Sci. 1968 Aug;46(4):375–396. doi: 10.1038/icb.1968.32. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Cerottini J. C. The immunogenicity of antigen bound to the plasma membrane of macrophages. J Exp Med. 1970 Apr 1;131(4):711–725. doi: 10.1084/jem.131.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]


