Abstract
Experimental glomerulonephritis (EGN) was produced in Sprague-Dawley rats with a single intradermal injection of a renal tubular fraction called Fx1A. Presence of disease was confirmed by proteinuria and by light and electron microscopic changes. This antigen and also the non-nephritogenic antigen, Fx1B, were used to study cellular immunity in nephritic and three groups of control rats.
64 per cent (9/14) of the Fx1A immunized rats gave delayed hypersensitivity skin tests to Fx1A. The difference between the experimental and the three control groups was highly significant.
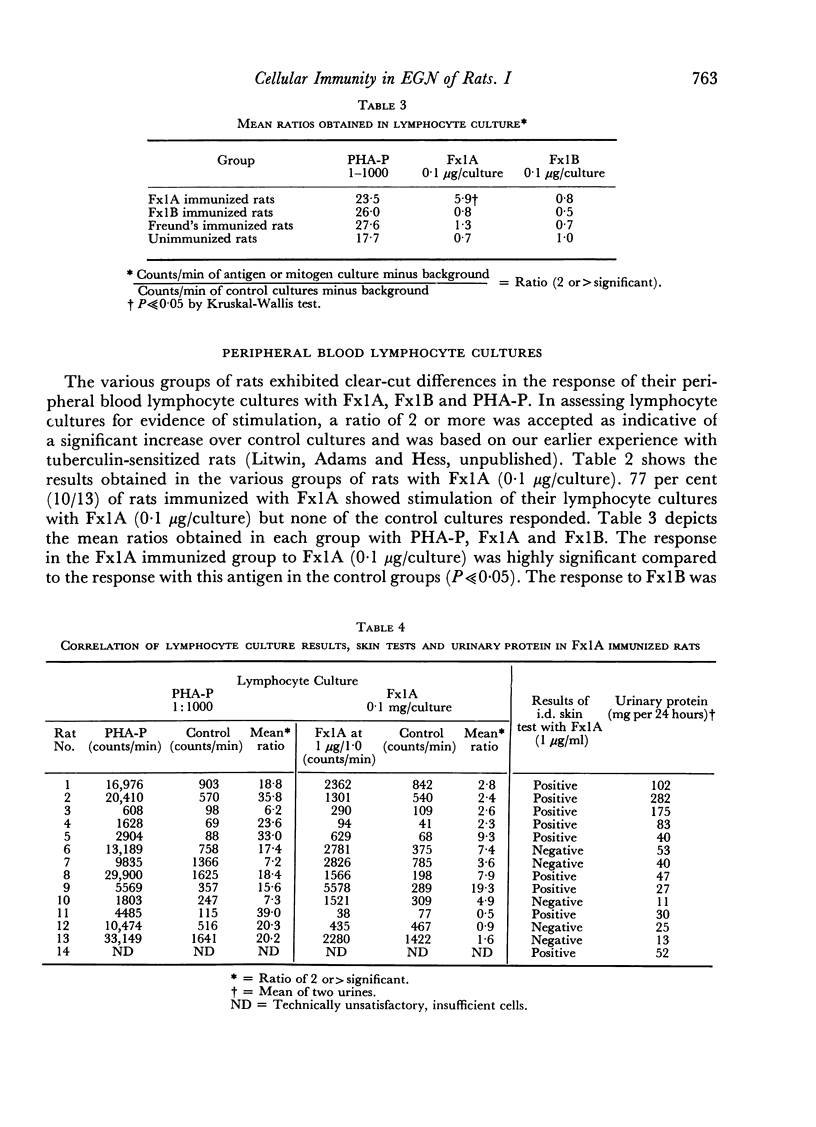
Peripheral blood lymphocyte culture responses to Fx1A, Fx1B, phytohaemagglutinin-P, were studied in the nephritic and control groups. 77 per cent (10/13) of Fx1A immunized animals showed stimulation of their lymphocyte cultures with Fx1A. None of the control groups showed any significant stimulation.
These experiments have demonstrated cellular immunity in EGN and support the possibility that these mechanisms may play a role in the production of this disease.
Full text
PDF




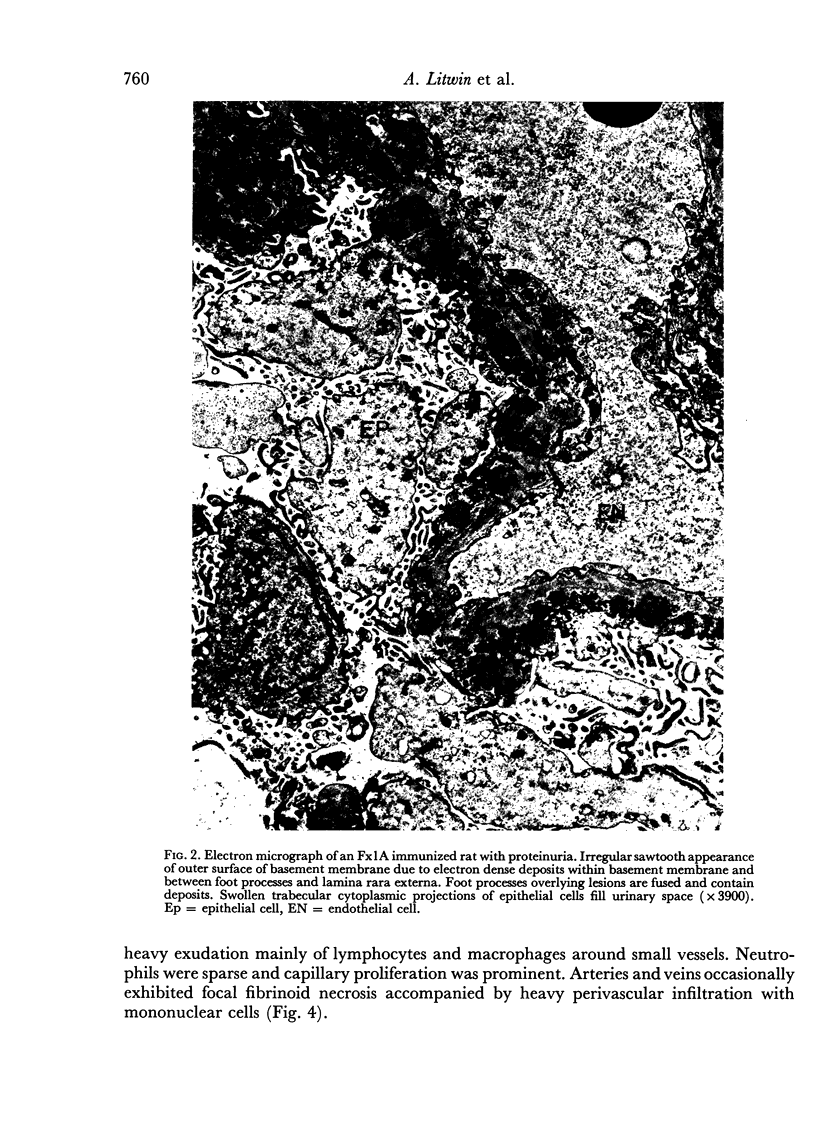
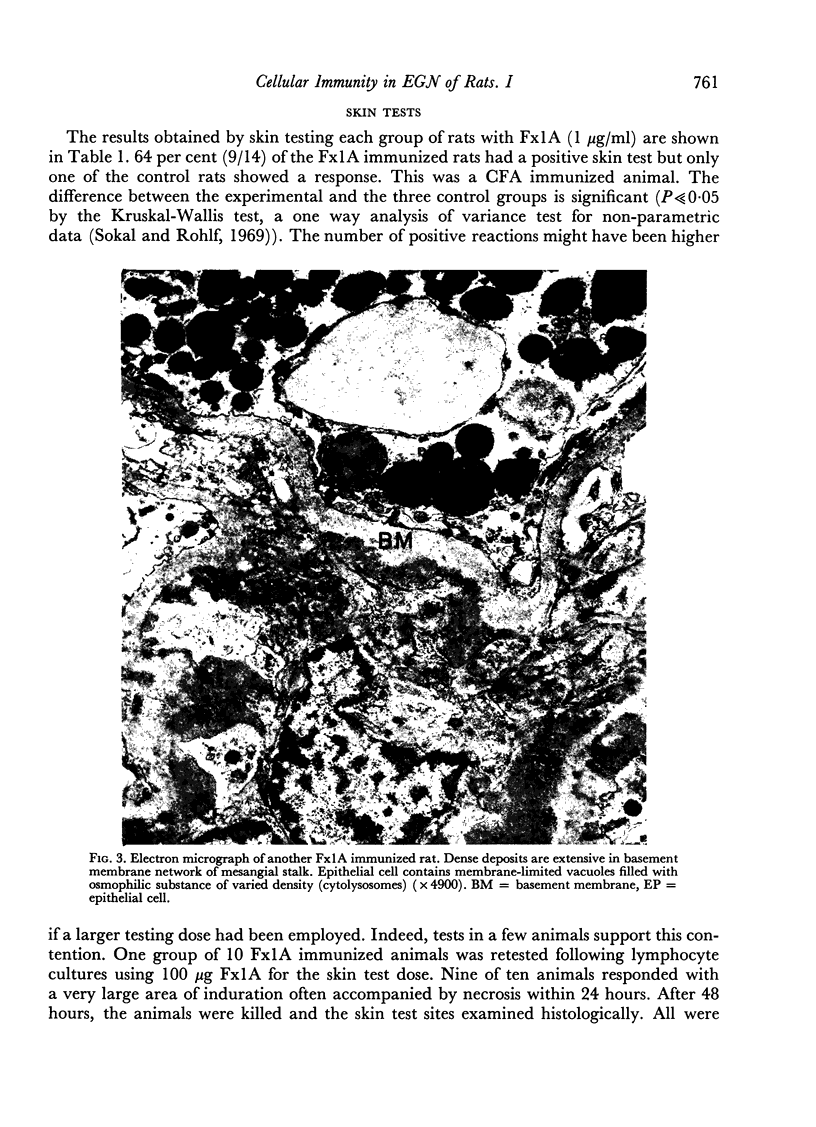
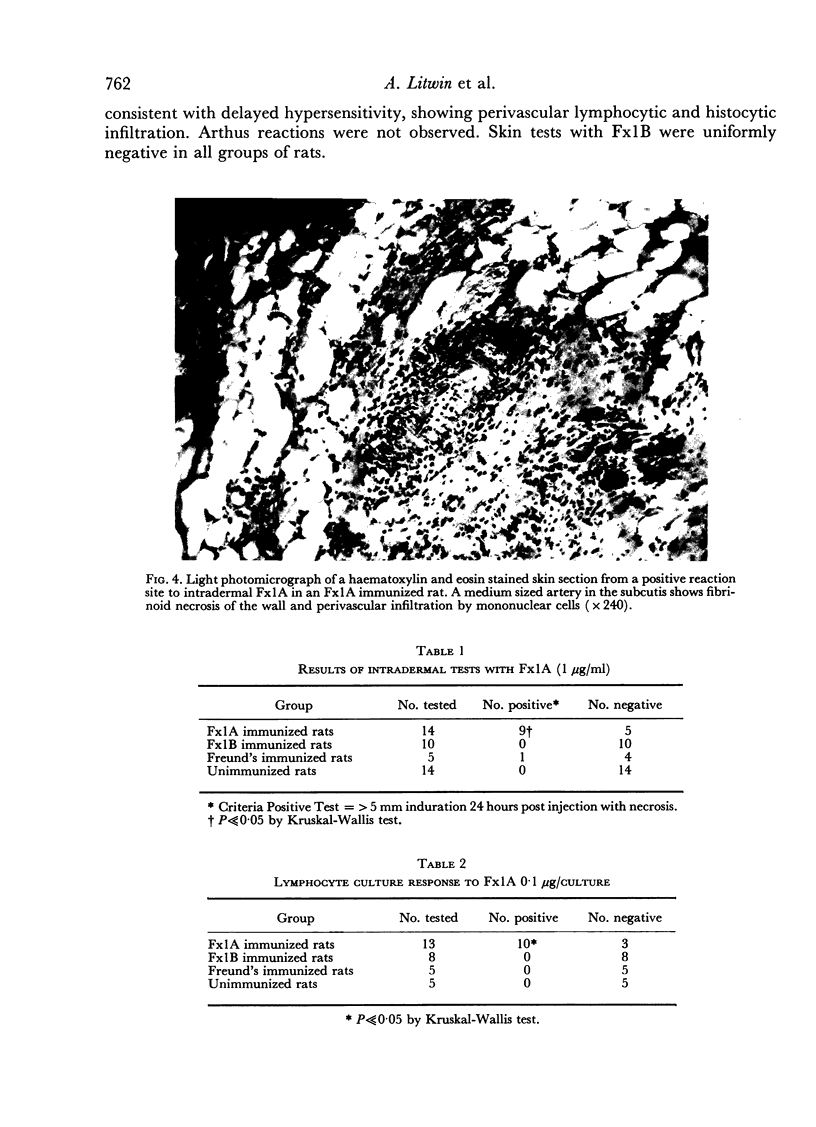



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benezra D., Gery I., Davies A. M. The relationship between lymphocyte transformation and immune responses. II. Correlations between transformation and humoral and cellular immune responses. Clin Exp Immunol. 1969 Jul;5(1):155–161. [PMC free article] [PubMed] [Google Scholar]
- Brown P. C., Glynn L. E., Holborow E. J. The dual necessity for delayed hypersensitivity and circulating antibody in the pathogenesis of experimental allergic orchitis in guinea-pigs. Immunology. 1967 Sep;13(3):307–314. [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. I. Identification and isolation of the pathogenetic antigen. J Exp Med. 1968 Mar 1;127(3):555–572. doi: 10.1084/jem.127.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune-complex pathogenesis of experimental allergic glomerulonephritis. Science. 1967 Mar 17;155(3768):1432–1434. doi: 10.1126/science.155.3768.1432. [DOI] [PubMed] [Google Scholar]
- Glassock R. J., Edgington T. S., Watson J. I., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. II. The pathogenetic mechanism. J Exp Med. 1968 Mar 1;127(3):573–588. doi: 10.1084/jem.127.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe W. E. An in vitro demonstration of cellular sensitivity in experimental autoimmune nephrosis in rats. Proc Soc Exp Biol Med. 1968 Apr;127(4):1217–1222. doi: 10.3181/00379727-127-32914. [DOI] [PubMed] [Google Scholar]
- Grupe W. E., Kaplan M. H. Demonstration of an antibody to proximal tubular antigen in the pathogenesis of experimental autoimmune nephrosis in rats. J Lab Clin Med. 1969 Sep;74(3):400–409. [PubMed] [Google Scholar]
- HESS E. V., ASHWORTH C. T., ZIFF M. Transfer of an autoimmune nephrosis in the rat by means of lymph node cells. J Exp Med. 1962 Feb 1;115:421–438. doi: 10.1084/jem.115.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- HEYMANN W., HUNTER J. L., HACKEL D. B., CUPPAGE F. Transfer of experimental auto-immune nephrosis in rats. Proc Soc Exp Biol Med. 1962 Dec;111:568–573. doi: 10.3181/00379727-111-27857. [DOI] [PubMed] [Google Scholar]
- Holm G. In vitro cytotoxic effects of lymphoid cells from rats with experimental autoimmune nephrosis. Clin Exp Immunol. 1966 Jan;1(1):45–60. [PMC free article] [PubMed] [Google Scholar]
- Mills J. A. The immunologic significance of antigen induced lymphocyte transformation in vitro. J Immunol. 1966 Aug;97(2):239–247. [PubMed] [Google Scholar]
- Oppenheim J. J., Wolstencroft R. A., Gell P. G. Delayed hypersensitivity in the guinea-pig to a protein-hapten conjugate and its relationship to in vitro transformation of lymph node, spleen, thymus and peripheral blood lymphocytes. Immunology. 1967 Jan;12(1):89–102. [PMC free article] [PubMed] [Google Scholar]
- Stulbarg M., Schlossman S. F. The specificity of antigen-induced thymidine-2-14C incorporation into lymph node cells frrom sensitized animals. J Immunol. 1968 Oct;101(4):764–769. [PubMed] [Google Scholar]
- Wilson D. B. Quantitative studies on the mixed lymphocyte interaction in rats. I. Conditions and parameters of response. J Exp Med. 1967 Oct 1;126(4):625–654. doi: 10.1084/jem.126.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]