Abstract
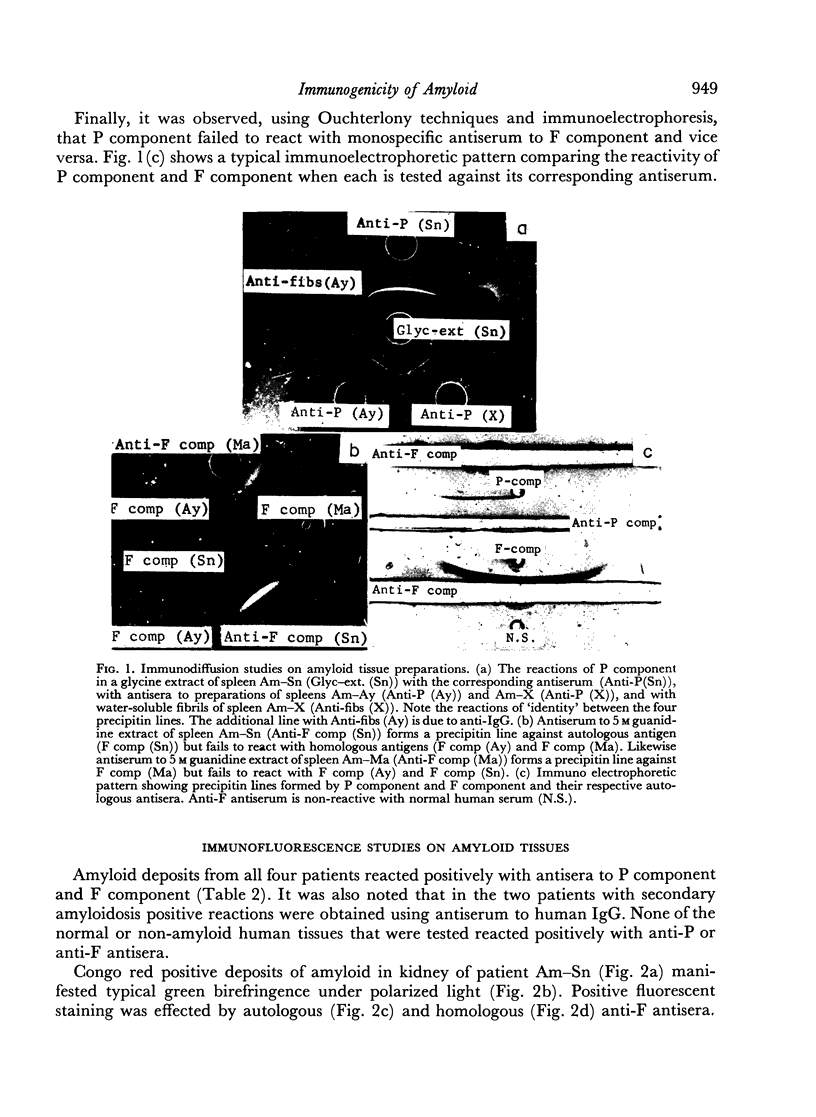
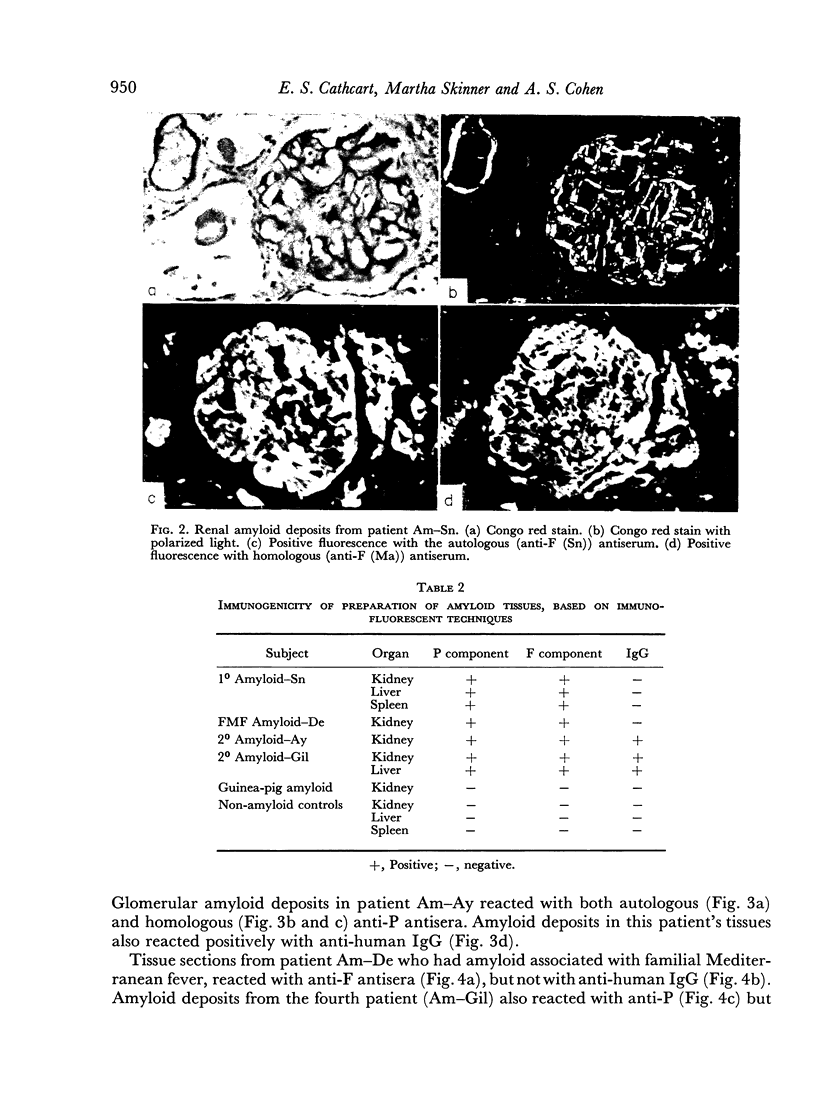
Human amyloid appears to contain two immunogenic components, the P (plasma) component and the F (fibril) component. Antisera to P component react with both autologous and homologous antigens by immunodiffusion and fluorescent antibody techniques; antisera to F component react with autologous antigens by immunodiffusion and fluorescent antibody techniques, but react with homologous antigens only by the fluorescent antibody method. Antisera to both P component and F component fail to react with non-amyloid human tissues as well as amyloid deposits in guinea-pig tissues. These results point to heterogeneity in the antigenic determinants of human amyloid and possibly greater inter-species heterogeneity of amyloid.
Full text
PDF



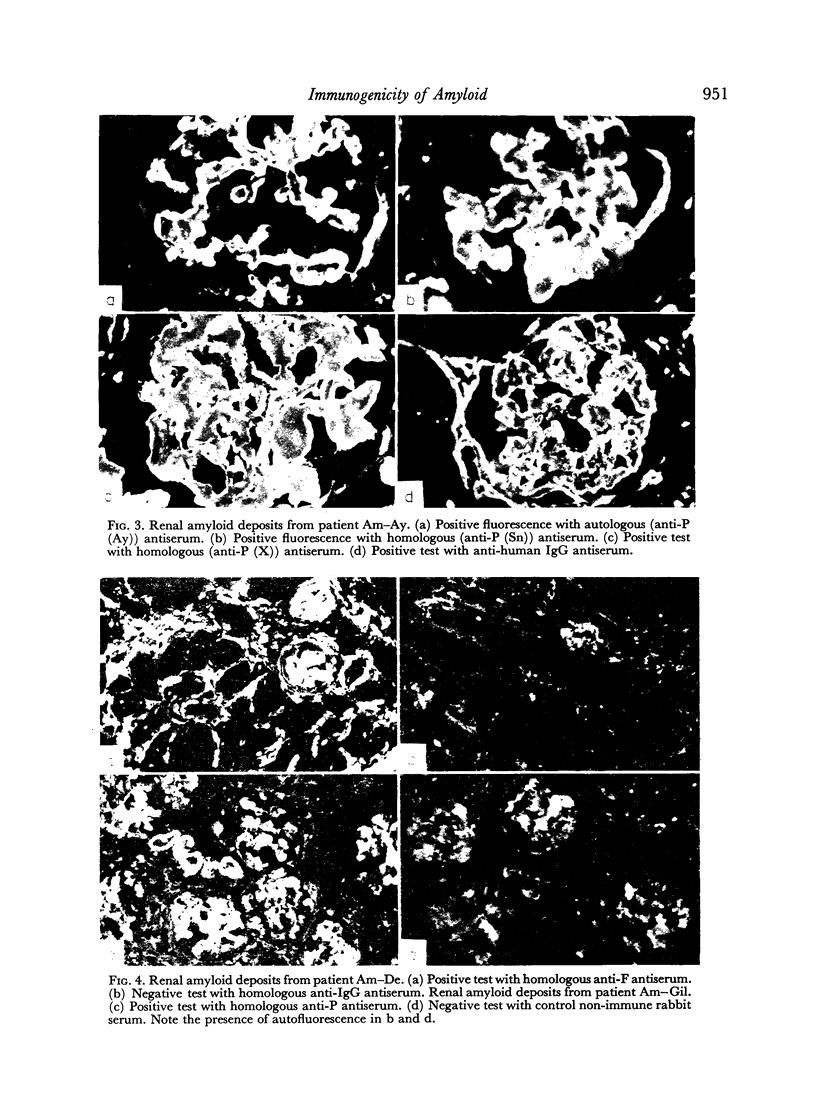



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bladen H. A., Nylen M. U., Glenner G. G. The ultrastructure of human amyloid as revealed by the negative staining technique. J Ultrastruct Res. 1966 Mar;14(5):449–459. doi: 10.1016/s0022-5320(66)80075-8. [DOI] [PubMed] [Google Scholar]
- CATHCART E. S., COMERFORD F. R., COHEN A. S. IMMUNOLOGIC STUDIES ON A PROTEIN EXTRACTED FROM HUMAN SECONDARY AMYLOID. N Engl J Med. 1965 Jul 15;273:143–146. doi: 10.1056/NEJM196507152730306. [DOI] [PubMed] [Google Scholar]
- COHEN A. S., CALKINS E. Electron microscopic observations on a fibrous component in amyloid of diverse origins. Nature. 1959 Apr 25;183(4669):1202–1203. doi: 10.1038/1831202a0. [DOI] [PubMed] [Google Scholar]
- COHEN A. S., CALKINS E. THE ISOLATION OF AMYLOID FIBRILS AND A STUDY OF THE EFFECT OF COLLAGENASE AND HYALURONIDASE. J Cell Biol. 1964 Jun;21:481–486. doi: 10.1083/jcb.21.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart E. S., Cohen A. S. The relation between isolated human amyloid fibrils and human gamma-globulin and its subunits. J Immunol. 1966 Feb;96(2):239–244. [PubMed] [Google Scholar]
- Cathcart E. S., Wollheim F. A., Cohen A. S. Plasma protein constituents of amyloid fibrils. J Immunol. 1967 Aug;99(2):376–385. [PubMed] [Google Scholar]
- DIXON F. J., VAZQUEZ J. J. Immunohistochemical analysis of amyloid by the fluorescence technique. J Exp Med. 1956 Nov 1;104(5):727–736. doi: 10.1084/jem.104.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin E. C., Pras M. Immunologic studies of water-soluble human amyloid fibrils. Comparative studies of eight amyloid preparations. J Exp Med. 1969 Oct 1;130(4):797–808. doi: 10.1084/jem.130.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWITZ R. E., STUYVESANT V. W., WIGMORE W., TATTER D. FIBRINOGEN AS A COMPONENT OF AMYLOID. Arch Pathol. 1965 Mar;79:238–244. [PubMed] [Google Scholar]
- LACHMANN P. J., MULLER-EBERHARD H. J., KUNKEL H. G., PARONETTO F. The localization of in vivo bound complement in tissue section. J Exp Med. 1962 Jan 1;115:63–82. doi: 10.1084/jem.115.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LETTERER E., CAESAR R., VOGT A. [Studies on electronoptic and immunomorphological structure of amyloid]. Dtsch Med Wochenschr. 1960 Oct 28;85:1909–1910. doi: 10.1055/s-0028-1112672. [DOI] [PubMed] [Google Scholar]
- MCDEVITT H. O., PETERS J. H., POLLARD L. W., HARTER J. G., COONS A. H. PURIFICATION AND ANALYSIS OF FLUORESCEIN-LABELED ANTISERA BY COLUMN CHROMATOGRAPHY. J Immunol. 1963 Apr;90:634–642. [PubMed] [Google Scholar]
- MELLORS R. C., ORTEGA L. G. Analytical pathology. III. New observations on the pathogenesis of glomerulonephritis, lipid nephrosis, periarteritis nodosa, and secondary amyloidosis in man. Am J Pathol. 1956 May-Jun;32(3):455–499. [PMC free article] [PubMed] [Google Scholar]
- OSSERMAN E. F., TAKATSUKI K., TALAL N. MULTIPLE MYELOMA I. THE PATHOGENESIS OF "AMYLOIDOSIS. Semin Hematol. 1964 Jan;1:3–85. [PubMed] [Google Scholar]
- OVARY Z. Immediate reactions in the skin of experimental animals provoked by antibody-antigen interaction. Prog Allergy. 1958;5:459–508. [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M., Zucker-Franklin D., Rimon A., Franklin E. C. Physical, chemical, and ultrastructural studies of water-soluble human amyloid fibrils. Comparative analyses of nine amyloid preparations. J Exp Med. 1969 Oct 1;130(4):777–796. doi: 10.1084/jem.130.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINDERKNECHT H. Ultra-rapid fluorescent labelling of proteins. Nature. 1962 Jan 13;193:167–168. doi: 10.1038/193167b0. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Schultz R. T., Calkins E., Milgrom F., Witebsky E. Association of gamma globulin with amyloid. Am J Pathol. 1966 Jan;48(1):1–17. [PMC free article] [PubMed] [Google Scholar]
- Sellin D., Haferkamp O. Bence Jones Protein und Amyloid. Untersuchungen zum Zusammenhang von natürlicherweise aufretendem menschlichem Antikörper gegen Bence Jones Protein und von Bence Jones Protein mit Amyloid. Z Gesamte Exp Med. 1968;147(3):173–189. [PubMed] [Google Scholar]
- Sri Ram J., DeLellis R. A., Glenner G. G. Amyloid. IV. Is human amyloid immunogenic? Int Arch Allergy Appl Immunol. 1968;34(3):269–282. [PubMed] [Google Scholar]
- VOGT A., KOCHEM H. G. [Histo-serological studies with fluorescein-labeled anticomplement. Demonstration of complement-binding substances in amyloid]. Z Zellforsch Mikrosk Anat. 1960;52:640–652. [PubMed] [Google Scholar]